Abstract
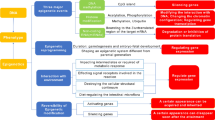
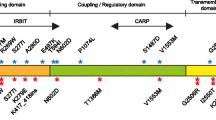
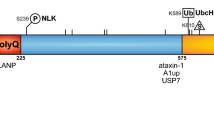
The spinocerebellar ataxias (SCAs) are a clinically, genetically, and neuropathologically heterogeneous group of neurological disorders defined by variable degrees of cerebellar ataxia often accompanied by additional cerebellar and non-cerebellar symptoms that, in many cases, defy differentiation based on clinical characterisation alone. The clinical symptoms are triggered by neurodegeneration of the cerebellum and its relay connexions. The current identification of at least 43 SCA subtypes and the causative molecular defects in 27 of them refine the clinical diagnosis, provide molecular testing of at risk, a/pre-symptomatic, prenatal or pre-implantation and facilitate genetic counselling. The recent discovery of new causative SCA genes along with the respective scientific advances is uncovering high complexity and altered molecular pathways involved in the mechanisms by which the mutant gene products cause pathogenesis. Fortunately, the intensive ongoing clinical and neurogenetic research together with the applied molecular approaches is sure to yield scientific advances that will be translated into developing effective treatments for the spinocerebellar ataxias and other similar neurological conditions.

Similar content being viewed by others
Abbreviations
- ADCA:
-
Autosomal dominant spinocerebellar ataxia
- ADHD:
-
Attention deficit/hyperactivity disorder
- ADNOA:
-
Ataxia with deafness, narcolepsy, and optic atrophy
- ADSA:
-
Autosomal dominant sensory ataxia
- AFG3L2:
-
ATPase family gene 3-like 2
- ARP1:
-
Actin-related protein-1
- ATN1:
-
Atrophin-1
- ATXN1:
-
Ataxin-1
- ATXN2:
-
Ataxin-2
- ATXN3:
-
Ataxin-3
- ATXN8:
-
Ataxin-8
- ATXN8OS:
-
Ataxin-8 opposite strand
- Ca2+ :
-
Calcium ion
- BEAN1:
-
Brain expressed associated NEDD4
- CACNA1A:
-
Calcium channel, voltage-dependent, P/Q type, alpha 1A subunit
- CACNB4:
-
Calcium channel, voltage-dependent, subunit beta 4
- CAG:
-
DNA sequence coding for glutamine
- DRPLA:
-
Dentatorubral-pallidoluysian atrophy
- EA:
-
Episodic ataxia
- EAAT1:
-
Excitatory amino acid transporter 1
- EMQN:
-
European Molecular Quality Genetics network
- FGF14:
-
Fibroblast growth factor 14
- HGNC:
-
HUGO Gene Nomenclature Committee
- IFRD1:
-
Interferon-related developmental regulator gene 1
- INAS:
-
Inventory of non-ataxia symptoms
- ITPR:
-
Inositol triphosphate receptor
- K+ :
-
Potassium ion
- KCNA1:
-
Potassium voltage-gated channel, shaker-related subfamily, member 1
- KCNC3:
-
Potassium voltage-gated channel subfamily C member 3
- MJD:
-
Machado–Joseph disease
- NOP56:
-
Ribonucleoprotein homolog yeast
- OECD:
-
Organisation for Economic Co-operation and Development
- PDYN:
-
Prodynorphin
- PPP2R2B:
-
Serine/threonine protein phosphatase 2 (formerly 2A) 55 kDa regulatory subunit B beta isoform
- PRKCG:
-
Protein kinase C gamma
- SARA:
-
Scale for the Assessment and Rating of Ataxia
- SCA:
-
Spinocerebellar ataxia
- SCN8A:
-
Sodium channel, voltage gated, type VIII, alpha subunit
- SLC1A3:
-
Solute carrier family 1 member 3
- SPAX1:
-
Autosomal dominant spastic ataxia
- SPTBN2:
-
Beta-III spectrin
- TBP:
-
TATA box binding protein
- TK2:
-
Thymidine kinase 2
- TTBK2:
-
Tau tubulin kinase-2
References
Matilla-Dueñas A, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain. 2006;129:1357–70.
Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–94.
Matilla-Dueñas A, Sanchez I, Corral-Juan M, Davalos A, Alvarez R, Latorre P. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum. 2010;9:148–66.
Matilla-Dueñas A. Machado–Joseph disease and other rare spinocerebellar ataxias. In: Ahmad S, editor. Neurodegenerative diseases. Austin: Landes Bioscience; 2012. p. 172–88.
Matilla-Dueñas A, Corral-Juan M, Volpini V, Sanchez I. The spinocerebellar ataxias: clinical aspects and molecular genetics. In: Ahmad SI, editor. Neurodegenerative diseases. Austin: Landes Bioscience; 2012. p. 351–74.
McKusick V (2010) Online Mendelian Inheritance in Man, OMIM (TM). McKusick-Nathans Institute of Genetic Medicine Johns Hopkins University (Baltimore, MD) and National Center for Biotechnology Information, National Library of Medicine, Bethesda, MD
Pagon RA, Bird TC, Dolan CR, Stephenson DA. Gene reviews [internet]. Seattle: University of Washington; 1993–2010.
Klockgether T. The clinical diagnosis of autosomal dominant spinocerebellar ataxias. Cerebellum. 2008;7:101–5.
Tsuji S, Onodera O, Goto J, Nishizawa M. Sporadic ataxias in Japan—a population-based epidemiological study. Cerebellum. 2008;7:189–97.
Erichsen AK, Koht J, Stray-Pedersen A, Abdelnoor M, Tallaksen CM. Prevalence of hereditary ataxia and spastic paraplegia in southeast Norway: a population-based study. Brain J Neurol. 2009;132:1577–88.
Velazquez Perez L, Cruz GS, Santos Falcon N, Almaguer Mederos LE, Escalona Batallan K, Rodriguez Labrada R, et al. Molecular epidemiology of spinocerebellar ataxias in Cuba: insights into SCA2 founder effect in Holguin. Neurosci Lett. 2009;454:157–60.
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20.
Schmitz-Hubsch T, Fimmers R, Rakowicz M, Rola R, Zdzienicka E, Fancellu R, et al. Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology. 2011;74:678–84.
Schmitz-Hubsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, et al. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology. 2008;71:982–9.
Chan E, Charles P, Ribai P, Goizet C, Marelli C, Vincitorio CM, et al. Quantitative assessment of the evolution of cerebellar signs in spinocerebellar ataxias. Mov Disord. 2011;26:534–8.
Jacobi H, Bauer P, Giunti P, Labrum R, Sweeney MG, Charles P, et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study. Neurology. 2011;77(11):1035–41.
Orr H, M-y C, Banfi S, Kwiatkowski Jr TJ, Servadio A, Beaudet AL, et al. Expansion of an unstable trinucleotide (CAG) repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4:221–6.
Kobayashi H, Abe K, Matsuura T, Ikeda Y, Hitomi T, Akechi Y, et al. Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am J Hum Genet. 2011;89:121–30.
Sequeiros J, Martindale J, Seneca S. EMQN Best Practice Guidelines for molecular genetic testing of SCAs. Eur J Hum Genet. 2010;18:1173–6.
Sequeiros J, Seneca S, Martindale J. Consensus and controversies in best practices for molecular genetic testing of spinocerebellar ataxias. Eur J Hum Genet. 2010;18:1188–95.
Ogawa M. Pharmacological treatments of cerebellar ataxia. Cerebellum. 2004;3:107–11.
Manto M, Marmolino D. Cerebellar ataxias. Curr Opin Neurol. 2009;22:419–29.
Trujillo-Martin MM, Serrano-Aguilar P, Monton-Alvarez F, Carrillo-Fumero R. Effectiveness and safety of treatments for degenerative ataxias: a systematic review. Mov Disord. 2009;24:1111–24.
Goizet C, Lesca G, Durr A. Presymptomatic testing in Huntington’s disease and autosomal dominant cerebellar ataxias. Neurology. 2002;59:1330–6.
Watson MJ. Systematic review of the effectiveness of physiotherapy for cerebellar dysfunction. Clin Rehabil. 2009;23:764–5.
Ilg W, Synofzik M, Brotz D, Burkard S, Giese MA, Schols L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73:1823–30.
Missaoui B, Thoumie P. How far do patients with sensory ataxia benefit from so-called “proprioceptive rehabilitation”? Neurophysiol Clin. 2009;39:229–33.
Corral-Juan M, Corral J, San Nicolás H, Volpini V, Matilla-Dueñas A. Genetics of the autosomal dominant spinocerebellar ataxias. In: eLS. Chichester: Wiley; 2011. doi:10.1002/9780470015902.a0006076.
Further Reading
Matilla-Dueñas A. Machado–Joseph disease and other rare spinocerebellar ataxias. In: Ahmad S, editor. Neurodegenerative diseases. Austin: Landes Bioscience; 2012. p. 172–88.
Matilla-Dueñas A, Corral-Juan M, Volpini V, Sanchez I. The spinocerebellar ataxias: clinical aspects and molecular genetics. In: Ahmad SI, editor. Neurodegenerative diseases. Austin: Landes Bioscience; 2012. p. 351–74.
Matilla-Dueñas A, Serrano C, Alvarez R, Ivánovic-Barbeito YP, Latorre P, Genís D. Novel therapeutic challenges in cerebellar diseases. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders. New York: Springer; 2012.
Subramony SH. Overview of autosomal dominant ataxias. In: Subramony SH, Dürr A, editors. Ataxic disorders. Handbook of clinical neurology, vol. 103. Edinburgh: Elsevier; 2012. 3rd series.
Acknowledgments
Dr. Ivelisse Sanchez’s helpful comments and suggestions are kindly acknowledged. Dr. Antoni Matilla’s scientific research on ataxias is funded by the Spanish Ministry of Science and Innovation (BFU2008-00527/BMC), the Carlos III Health Institute (CP08/00027), the Latin American Science and Technology Development Programme (CYTED) (RIBERMOV, 210RT0390), the European Commission (EUROSCA project, LHSM-CT-2004-503304), and the Fundació de la Marató de TV3 (Televisió de Catalunya, 100730). We are indebted to the Spanish Ataxia Association (FEDAES), the Spanish Federation for Rare Diseases (FEDER), and the ataxia patients for their continuous support and motivation. Antoni Matilla is a Miguel Servet Investigator in Neurosciences of the Spanish National Health System.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matilla-Dueñas, A. The Ever Expanding Spinocerebellar Ataxias. Editorial. Cerebellum 11, 821–827 (2012). https://doi.org/10.1007/s12311-012-0376-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0376-4