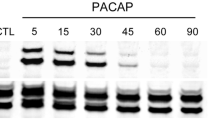
Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuropeptide, regulates a wide variety of cellular functions, but little is known about its role in neuronal cell migration. Recent studies revealed that PACAP has short-term, cortical layer-specific effects on neuronal cell migration. In this article, we focus on the role of PACAP in controlling the migration of cerebellar granule cells.




Similar content being viewed by others
References
Rakic P (1990) Principles of neuronal cell migration. Experientia 46:882–891
Kriegstein AR, Noctor SC (2004) Patterns of neuronal migration in the embryonic cortex. Trends Neurosci 27:392–9
Miale IL, Sidman RL (1961) An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol 4:277–296
Rakic P (1988) Defects of neuronal migration and the pathogenesis of cortical malformations. Prog Brain Res 73:15–37
Gressens P (2006) Pathogenesis of migration disorders. Curr Opin Neurol 19:135–14
Sheen VL, Ferland RJ, Harney M, Hill RS, Neal J, Banham AH et al (2006) Impaired proliferation and migration in human Miller-Dieker neuronal precursors. Ann Neurol 60:137–144
Rakic P, Cameron SR, Komuro H (1994) Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol 4:63–69
Bagri A, Tessier-Lavigne M (2002) Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance. Adv Exp Med Biol 515:13–31
Marin O, Rubenstein JL (2003) Cell migration in the forebrain. Annu Rev Neurosci 26:441–483
Cameron DB, Galas L, Jiang Y, Raoult E, Vaudry D, Komuro H (2007) Cerebellar cortical-layer-specific control of neuronal migration by pituitary adenylate cyclase-activating polypeptide. Neuroscience 146:697–712
Botia B, Basille M, Allais A, Raoult E, Falluel-Morel A, Galas L et al (2007) Neurotrophic effects of PACAP in the cerebellar cortex. Peptides 28:1746–1752
Falluel-Morel A, Vaudry D, Aubert N, Galas L, Benard M, Basille M et al (2005) Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proc Natl Acad Sci U S A 102:2637–2642
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52:269–324
Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K et al (1990) Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun 170:643–648
Pisegna JR, Wank SA (1993) Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci U S A 90:6345–6349
Takeda K, Okamura T, Hasegawa T (1989) Sibs with tetrasomy 18p born to a mother with trisomy 18p. J Med Genet 26:195–197
Salihu HM, Boos R, Schmidt W (1997) Antenatally detectable markers for the diagnosis of autosomally trisomic fetuses in at-risk pregnancies. Am J Perinatol 14:257–261
Lang B, Song B, Davidson W, MacKenzie A, Smith N, McCaig CD et al (2006) Expression of the human PAC1 receptor leads to dose-dependent hydrocephalus-related abnormalities in mice. J Clin Invest 116:1924–1934
Allais A, Burel D, Issac ER, Gray SL, Basille M, Ravni A et al (2007) Altered cerebellar development in mice lacking pituitary adenylate cyclase-activating polypeptide. Eur J NeuroSci 25:2604–2618
Komuro H, Yacubova E (2003) Recent advances in cerebellar granule cell migration. Cell Mol Life Sci 60:1084–1098
Yacubova E, Komuro H (2003) Cellular and molecular mechanisms of cerebellar granule cell migration. Cell Biochem Biophys 37:213–234
Komuro H, Yacubova E, Yacubova E, Rakic P (2001) Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci 21:527–540
Komuro H, Rakic P (1995) Dynamics of granule cell migration: a confocal microscopic study in acute cerebellar slice preparations. J Neurosci 15:1110–1120
Komuro H, Rakic P (1998) Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci 18:1478–1490
Jiang Y, Kumada T, Cameron DB, Komuro H (2008) Cerebellar granule cell migration and the effects of alcohol. Dev Neurosci 30:7–23
Yacubova E, Komuro H (2002) Stage-specific control of neuronal migration by somatostatin. Nature 415:77–81
Komuro H, Rakic P (1993) Modulation of neuronal migration by NMDA receptors. Science 260:95–97
Nielsen HS, Hannibal J, Fahrenkrug J (1998) Expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the postnatal and adult rat cerebellar cortex. NeuroReport 9:2639–2642
Basille M, Vaudry D, Coulouarn Y, Jegou S, Lihrmann I, Fournier A et al (2000) Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J Comp Neurol 425:495–509
Basille M, Cartier D, Vaudry D, Lihrmann I, Fournier A, Freger P et al (2006) Localization and characterization of pituitary adenylate cyclase-activating polypeptide receptors in the human cerebellum during development. J Comp Neurol 496:468–478
Basille M, Gonzalez BJ, Leroux P, Jeandel L, Fournier A, Vaudry H (1993) Localization and characterization of PACAP receptors in the rat cerebellum during development: evidence for a stimulatory effect of PACAP on immature cerebellar granule cells. Neuroscience 57:329–338
Basille M, Gonzalez BJ, Fournier A, Vaudry H (1994) Ontogeny of pituitary adenylate cyclase-activating polypeptide (PACAP) receptors in the rat cerebellum: a quantitative autoradiographic study. Dev Brain Res 82:81–89
Basille M, Vaudry D, Coulouarn Y, Jegou S, Lihrmann I, Fournier H et al (2000) Distribution of PACAP receptor mRNAs and PACAP binding sites in the rat brain during development. Ann N Y Acad Sci 921:359–369
Mei YA (1999) High-voltage-activated calcium current and its modulation by dopamine D4 and pituitary adenylate cyclase-activating polypeptide receptors in cerebellar granule cells. Chung Kuo Yao Li Hsueh Pao 20:3–9
Basille M, Gonzalez BJ, Desrues L, Demas M, Fournier A, Vaudry H (1995) Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates adenylyl cyclase and phospholipase C activity in rate cerebellar neuroblasts. J Neurochem 65:1318–1324
Komuro H, Rakic P (1998) Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol 37:110–130
Kumada T, Komuro H (2004) Completion of neuronal migration regulated by loss of Ca2+ transients. Proc Natl Acad Sci U S A 101:8479–8484
Kumada T, Lakshmana MK, Komuro H (2006) Reversal of neuronal migration in a mouse model of fetal alcohol syndrome by controlling second-messenger signalings. J Neurosci 6:742–756
Dautzenberg FM, Hauger RL (2001) G-protein-coupled receptor kinase 3- and protein kinase C-mediated desensitization of the PACAP receptor type 1 in human Y-79 retinoblastoma cells. Neuropharmacology 40:394–407
Shintani N, Hashimoto H, Kunugi A, Koyama Y, Yamamoto K, Tomimoto S et al (2000) Desensitization, surface expression, and glycosylation of a functional, epitope-tagged type I PACAP (PAC(1)) receptor. Biochem Biophys Acta 1509:195–202
Chuang TT, Paolucci L, Blasi A (1996) Inhibition of G protein-coupled receptor kinase subtypes by Ca2+/calmodulin. J Biol Chem 71:28691–28696
Favit A, Scapagnini U, Canonico PL (1995) Pituitary adenylate cyclase-activating polypeptide activates different signal transducing mechanisms in cultured cerebellar granule cells. Neuroendocrinology 61:377–382
Komuro H, Rakic P (1996) Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron 17:275–285
Kumada T, Jiang Y, Cameron DB, Komuro H (2007) How does alcohol impair neuronal migration? J Neurosci Res 85:465–470
Acknowledgements
We thank Y. Komuro for critically reading the manuscript. Work reported in this review was supported by a Pilot Research Award (PP1450) from the National Multiple Sclerosis Society and a grant (R01 ES015612) from National Institute of Environmental Health Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cameron, D.B., Raoult, E., Galas, L. et al. Role of PACAP in Controlling Granule Cell Migration. Cerebellum 8, 433–440 (2009). https://doi.org/10.1007/s12311-009-0121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-009-0121-9