Abstract
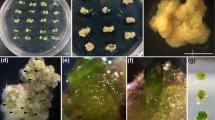
An efficient and simple procedure for inducing high frequency direct shoot organogenesis and somatic embryogenesis in lentil from cotyledonary node explants (without both the cotyledons) in response to TDZ alone is reported. TDZ at concentration lower than 2.0 μM induced shoot organogenesis whereas at higher concentration (2.5–15 μM) it caused a shift in regeneration from shoot organogenesis to somatic embryogenesis. The cotyledonary node and seedling cultures developed only shoots even at high concentrations of BAP and TDZ, respectively. TDZ at 0.5 and 5.0 μM was found to be optimal for inducing an average of 4–5 shoots per cotyledonary node in 93 % of the cultures and 55 somatic embryos in 68 % of the cultures, respectively. The somatic embryos were germinated when transferred to lower TDZ concentration (0.5–1.0 μM). The shoots were rooted on MS basal medium containing 2.5 μM IBA. The plantlets were obtained within 8 weeks from initiation of culture and were morphologically similar to seed-raised plants. The possible role of stress in thidiazuron induced somatic embryogenesis is discussed.
Similar content being viewed by others
Change history
22 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12298-022-01231-2
Abbreviations
- 2-iP:
-
2-isopentanyl adenine
- TDZ:
-
Thidiazuron
- AdS:
-
Adenine sulphate
- KIN:
-
Kinetin
- BAP:
-
6-benzyl aminopurine
- IBA:
-
Indole-3-butyric acid
Refrences
Amutha S, Muruganantham and Ganapathi A (2006). Thidiazuron induced high frequency axillary and adventitious shoot regeneration in Vigna radiata L. Wilczek. In Vitro Cell Dev Biol Plant 42: 26–30.
Bajaj YPS and Dhanju MS (1979). Regeneration of plants from apical meristem tips of some legumes. Curr Sci 84: 906–907.
Bates S, Preece JE and Navarrete NE (1992). TDZ stimulates shoots organogenesis in white ash (Fraximus americana L.). Plant Cell Tiss. Org. Cult. 31: 21–29.
Brunning JL and Kintz BL (1977). Computational hand book of statistics. 2nd edition, Scott, Foresman Glenview, CA.
Chaudhary D, Madanpotra S, Jaiwal R, Saini R, Kumar PA and Jaiwal PK (2006) Agrobacterium tumefaciens-mediated high frequency genetic transformation of Indian cowpea (Vigna unguiculata L. Walp.) Plant Sci. 172: 692–700.
FAOSTAT Report (2007). http://faostat.fao.org.
Gairi A and Rashid A (2004). Direct differentiation of somatic embryos on different regions of intact seedlings of Azadirachta in response to thidiazuron. J. Plant Physiol. 161: 1073–1077
Gill R and Saxena PK (1992). Direct somatic embryogenesis and regeneration of plants from seedling explants of peanut (Arachis hypogaea): promotive role of TDZ. Can. J. Bot. 70: 1186–1192
Gulati A, Schreyer P and Mc Hughen A (2001). Regeneration and micro-grafting of lentil shoots. In Vitro Cell. Dev. Biol. Plant 37: 798–802.
Gulati A and Mc Hughen A (2003). In vitro regeneration and genetic transformation of lentil. In: Applied Genetics of Leguminosae Biotechnology (Eds. Jaiwal PK and Singh RP), Kluwer Acad. Publ., The Netherlands, pp 133–147.
Jones MPA, Cao J, O’Brien, Murch SJ and Saxena P K (2007). The mode of action of thidiazuron: auxin, indoleamines and ion channels in the regeneration of Echinacea purpurea L. Plant Cell Rep. 26:1481–1490.
Ikeda-Iwai M, Umehara M, Satoh S and Kamada H (2003). Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J. 34:107–114.
Kamada H, Ishikawa K, Saga H and Harada H (1993). Induction of somatic embryogenesis in carrot by osmotic stress. Plant Tiss. Cult. Lett. 10: 38–44.
Kaneda Y, Tabei Y, Nishimura S, Harada K, Akhima T and Kitamura K (1977). Combination of thidiazuron and basal media with low salt concentrations increases the frequency of shoot organogenesis in soybean (Glycine max. L. Merr.) Plant Cell Rep. 17: 8–12.
Kiran G, Kaviraj CP, Jogeswar G, Kavikishor PB and Srinath Rao (2005). Direct and high frequency somatic embryogenesis and plant regeneration from hypocotyls of chickpea (Cicer arietinum L.), a grain legume. Curr Sci. 89: 1012–1018.
Kiyosue T, Takano K, Kamada H and Harada H (1990a). Induction of somatic embryogenesis by salt stress in carrot. Plant Tiss. Cult. Lett. 6: 162–164.
Kiyosue T, Takano K, Kamada H and Harada H (1990b). Induction of somatic embryogenesis in carrot by heavy metal ions. Can. J. Bot. 68: 2301–2303.
Lakshmanan P and Taji A (2000). Somatic embryogenesis in leguminous plants. Plant Biol. 2: 136–148.
Malik KA and Saxena PK (1992). Thidiazuron induces highfrequency shoot regeneration in intact seedlings of pea (Pisum sativum), chickpea (Cicer arietinum) and lentil (Lens culinaris). Australian J. Plant Physiol. 19: 731–740.
Mithila J, Hall JC Victor JMR and Saxena PK (2003). Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendt.). Plant Cell Rep. 21: 408–414.
Muehlbauer FJ, Cho S, Sarkar A, Mc Phee KE, Coyne CJ, Rajesh PN and Ford R (2006). Application of biotechnology in breeding lentil for resistance to biotic and abiotic stress. Euphytica 147:149–165.
Murashige T and Skoog F (1962). A revised medium for the rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Murch SJ, Victor JMR, Krishanraj S and Saxena PK (1999). The role of proline in thidiazuron — induced somatic embryogenesis of peanut. In vitro Cell. Dev. Biol. 35:102–105.
Murthy BNS, Murch SJ and Saxena PK (1995). Thidiazuroninduced somatic embryogenesis in intact seedlings of peanut (Arachis hypogea): Endogenous growth regulator levels and significance of cotyledon. Physiol. Plant. 94:268–276.
Murthy BNS, Murch SJ and Saxena PK (1998). Thidiazuron: a potent regulator of in vitro morphogenesis. In Vitro Cell. Dev. Biol. Plant 34:267–275.
Murthy BNS, Victor J, Singh RP, Fletcher RA and Saxena PK (1996). In vitro regeneration of chickpea (Cicer arietinum L.): stimulation of direct organogenesis and somatic embryogenesis by thidiazuron. Plant Growth Regul. 19:233–240.
Nishiwaki M, Fujino K, Koda Y, Masuda K and Kikuta Y (2000). Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759.
Polanco MC, Pelaez MI and Ruiz ML (1988). Factors affecting callus and shoot formation from in vitro cultures of Lens culinaris Medik. Plant Cell Tiss. Org. Cult. 15: 175–182.
Polanco MC and Ruiz ML (1997). Effect of benzylaminopurine on in vitro and in vivo root development in lentil (Lens culinaris Medik.). Plant Cell Rep. 17:22–26.
Sarker RH, Mustafa BM, Biswas A, Mahbub S, Nahra M, Hashem R and Hoque MI (2003). In vitro regeneration in lentil (Lens culinaris Medik.). Plant Tiss. Cult. 13:155–163.
Saxena PK and King J (1987). Morphogenesis in lentil: plant regeneration form callus cultures of Lens culinaris Medik. via somatic embryogenesis. Plant Sci. 52: 223–227.
Singh ND, Sahoo L, Sarin NB and Jaiwal PK (2003). The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan L. Millsp.) Plant Sci. 52: 223–227.
Singh RK and Raghuvanshi SS (1989). Plantlet regeneration from nodal segments and shoot tip derived explants of lentil. LENS Newsletter 16: 33–35.
Warkentin TD and Mc Hughen A (1993). Regeneration from lentil cotyledonary nodes and potential of this explant for transformation by Agrobacterium tumefaciens. LENS Newsletter 20: 26–28.
Williams DJ and Mc Hughen A (1986). Plant regeneration of the legume Lens culinaris Medik. (Lentil). in vitro. Plant Cell Tiss. Org. Cult. 7: 149–153.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chhabra, G., Chaudhary, D., Varma, M. et al. TDZ-induced direct shoot organogenesis and somatic embryogenesis on cotyledonary node explants of lentil (Lens culinaris Medik.). Physiol Mol Biol Plants 14, 347–353 (2008). https://doi.org/10.1007/s12298-008-0033-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-008-0033-z