Abstract
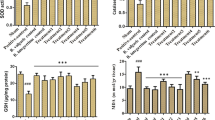
The effect of Emblica officinalis fruit extract (EFE) against alcohol-induced hepatic damage in rats was investigated in the present study. In vitro studies showed that EFE possesses antioxidant as well nitric oxide (NO) scavenging activity. In vivo administration of alcohol (5 g/kg b.wt/day) for 60 days resulted increased liver lipid peroxidation, protein carbonyls, nitrite plus nitrate levels. Alcohol administration also significantly lowers the activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione S-transferase and reduced glutathione as compared with control rats. Administration of EFE (250 mg/kg body weight) to alcoholic rats significantly brought the plasma enzymes towards near normal level and also significantly reduced the levels of lipid peroxidation, protein carbonyls and restored the enzymic and non-enzymatic antioxidants level. This observation was supplemented by histopathological examination in liver. Our data indicate that the tannoid, flavonoid and NO scavenging compounds present in EFE may offer protection against free radical mediated oxidative stress in rat hepatocytes of animals with alcohol-induced liver injury.

Similar content being viewed by others
References
Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–38.
Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown—focus on adducts. Alcohol Res Health. 2003;27:285–90.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424.
Venkatraman A, Shiva S, Wigley A, Ulasova E, Shhieng D, Bailey SM, et al. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–73.
Xu BJ, Zheng YN, Sung CK. Natural medicines for alcoholism treatment: a review. Drug Alcohol Rev. 2005;24:525–36.
Khopde SM, Indira Priyadarsini K, Mohan H, Gawandi VB, Satav JG, Yakhmi JV, et al. Characterizing the antioxidant activity of Amla (Phyllanthus emblica) extract. Curr Sci. 2001;81:185–90.
Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG. Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC-DPPH* method. J Sep Sci. 2007;30:1250–4.
Scartezzini P, Antognoni F, Raggi MA, Poli F, Sabbioni C. Vitamin C content and antioxidant activity of the fruit and of the Ayurvedic preparation Emblica officinalis Gaertn. J Ethnopharmacol. 2006;104:113–8.
De S, Ravishankar B, Bhavsar GC. Plants with hepatoprotective activity—a review. Indian Drugs. 1993;30:355–63.
Jindal A, Soyal D, Sharma A, Goyal PK. Protective effect of an extract of Emblica officinalis against radiation-induced damage in mice. Integr Cancer Ther. 2009;8:98–105.
Kim HJ, Yokozawa T, Kim HY, Tohda C, Rao TP, Juneja LR. Influence of amla (Emblica officinalis Gaertn.) on hypercholesterolemia and lipid peroxidation in cholesterol-fed rats. J Nutr Sci Vitaminol Tokyo. 2005;51:413–8.
Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, Lailerd N, Suttajit M, Jaikang C, et al. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med Chem. 2009;5:139–47.
Suryanarayana P, Saraswat M, Petrash JM, Reddy GB. Emblica officinalis and its enriched tannoids delay streptozotocin-induced diabetic cataract in rats. Mol Vis. 2007;13:1291–7.
Yokozawa T, Kim HY, Kim HJ, Okubo T, Chu DC, Juneja LR. Amla (Emblica officinalis Gaertn.) prevents dyslipidaemia and oxidative stress in the ageing process. Br J Nutr. 2007;97:1187–95.
Al-Rehaily AJ, Al-Howiriny TA, Al-Sohaibani MO, Rafatullah S. Gastroprotective effects of ‘Amla’ Emblica officinalis on in vivo test models in rats. Phytomedicine. 2002;9:515–22.
Sai Ram M, Neetu D, Yogesh B, Anju B, Dipti P, Pauline T, et al. Cyto-protective and immunomodulating properties of Amla (Emblica officinalis) in lymphocytes: an in vitro study. J Ethnopharmacol. 2002;81:5–10.
Reddy VD, Padmavathi P, Varadacharyulu NC. Emblica officinalis protects against ethanol-induced liver mitochondrial dysfunction in rats. J Med Food. 2009;12:327–33.
Reddy VD, Padmavathi P, Paramahamsa M, Varadacharyulu NC. Modulatory role of Emblica officinalis against alcohol induced biochemical and biophysical changes in rat erythrocyte membranes. Food Chem Toxicol. 2009;47:1958–63.
Miller NJ, Castelluccio C, Tijburg L, Rice-Evans C. The antioxidant properties of theaflavins and their gallate esters—radical scavengers or metal chelaters? FEBS Lett. 1996;392:40–4.
Sreejayan N, Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–7.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8.
Reznick AZ, Packer L. Oxidative damage to proteins: spectroscopic method for carbonyl assay. Methods Enzymol. 1994;233:357–63.
Abei H. Catalase in vitro. Methods Enzymol. 1988;10:121–6.
Mishra PH, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5.
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90.
Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7.
Sastry KVH, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306:79–82.
Lowry OH, Rosebrough NJ, Farr AL, Randall R. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:263–75.
Nishizawa M, Kohno M, Nishimura M, Kitagawa A, Niwano Y. Non-reductive scavenging of 1, 1-diphenyl-2-picryl hydrazyl (DPPH) by peroxyradical: a useful method for quantitative analysis of peroxyradical. Chem Pharm Bull. 2005;53:714–6.
Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74.
Verma R, Chakraborty D. Emblica officinalis aqueous extract ameliorates ochratoxin-induced lipid peroxidation in the testis of mice. Acta Pol Pharm. 2008;65:187–94.
Angel C. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11.
Sillanaukee P. Laboratory markers of alcohol abuse. Alcohol Alcohol. 1996;31:613–6.
Wang JF, Greenberg SS, Spitzer JJ. Chronic alcohol administration stimulates nitric oxide formation in the rat liver with or without pretreatment by lipopolysaccharide. Alcohol Clin Exp Res. 1995;19:387–93.
Yuan GJ, Zhou XR, Gong ZJ, Zhang P, Sun XM, Zheng SH. Expression and activity of inducible nitric oxide synthase and endothelial nitric oxide synthase correlate with ethanol-induced liver injury. World J Gastroenterol. 2006;12:2375–81.
Seitz HK, Salaspuro M, Savolainen M, Haber P, Ishii H, Teschke R, et al. From alcohol toxicity to treatment. Alcohol Clin Exp Res. 2005;29:1341–50.
Pigeolot E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxide and oxygen derived radicals. Mech Ageing Dev. 1990;51:283–97.
Wilce MC, Parker MW. Structure and function of glutathione s-transferases. Biochim Biophys Acta. 1994;1205:1–18.
Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600.
Alin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179:267–70.
Gueeri H. Influence on prolonged ethanol intake on the level and turnover of alcohol and aldehyde dehydrogenase and glutathione. Adv Exp Med Biol. 1995;23:12–4.
Bhattacharya A, Ghosal S, Bhattacharya SK. Antioxidant activity of tannoid principles of Emblica officinalis (amla) in chronic stress induced changes in rat brain. Ind J Exp Biol. 2000;38:877–80.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Damodara Reddy, V., Padmavathi, P., Gopi, S. et al. Protective Effect of Emblica officinalis Against Alcohol-Induced Hepatic Injury by Ameliorating Oxidative Stress in Rats. Ind J Clin Biochem 25, 419–424 (2010). https://doi.org/10.1007/s12291-010-0058-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-010-0058-2