Abstract
Background
To clarify appropriate timing for magnetic resonance examination to predict pathological complete response to neoadjuvant chemotherapy for patients with human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancers in terms of tumor volume change.
Methods
Between September 2009 and December 2014, 113 women with HER2-positive (n = 51) and triple-negative (n = 62) invasive breast cancers undergoing neoadjuvant chemotherapy were enrolled. Patients with HER2-positive tumors underwent neoadjuvant chemotherapy with an anthracycline-based regimen followed by docetaxel with trastuzumab. Patients with triple-negative tumors underwent neoadjuvant chemotherapy with anthracycline-based (first in most cases) and taxane-based regimens. Magnetic resonance imaging was performed before neoadjuvant chemotherapy, between the regimens (midpoint examination), and after neoadjuvant chemotherapy (final examination). Response ratio of tumor volume was calculated and receiver-operating characteristic analyses for them for both subtypes were performed at the midpoint and final examinations.
Results
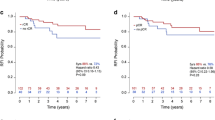
Twenty-eight women with HER2-positive tumors (54.9 %) and 29 women with triple-negative tumors (46.8 %) had pathological complete response. The response ratios were better in cases with pathological complete response than in those without (p = 0.0341, p < 0.0001). The area under the curve at the final examination was higher than that at the midpoint examination for HER2-positive tumors (p = 0.039); whereas for the triple-negative tumors, no significant difference between the two examinations was shown (p = 0.5218).
Conclusions
Magnetic resonance examination to predict pathological complete response would be feasible after completion of a regimen including trastuzumab for HER2-positive tumors and at the midpoint of neoadjuvant chemotherapy for triple-negative tumors.




Similar content being viewed by others
References
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22.
Von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Loo CE, Straver ME, Rodenhuis S, Muller SH, Wesseling J, Vrancken Peeters MJ, et al. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Oncol. 2011;29:660–9.
Loo CE, Teertstra HJ, Rodenhuis S, van de Vijver MJ, Hannemann J, Muller SH, et al. Dynamic contrast-enhanced MRI for prediction of breast cancer response to neoadjuvant chemotherapy: initial results. AJR. 2008;191:1331–8.
Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–72.
Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR. 2005;184:1774–81.
Yi Ann, Cho Nariya, Im Seock-Ah, Chang JM, Kim SJ, Moon HG, et al. Survival outcomes of breast cancer patients who receive neoadjuvant chemotherapy: association with dynamic contrast-enhanced MR imaging with computer-aided evaluation. Radiology. 2013;268:662–72.
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant chemotherapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–33.
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–31.
Sikov WM, Dizon DS, Strenger R, Legare RD, Theall KP, Graves TA, et al. Frequent pathologic complete responses in aggressive stages II to III breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: a Brown University Oncology Group Study. J Clin Oncol. 2009;27:3693–700.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Sonke GS, Mandjes IA, Holtkamp MJ, Schot M, van Werkhoven E, Wesseling J, et al. Paclitaxel, carboplatin, and trastuzumab in a neo-adjuvant regimen for HER2-positive breast cancer. Breast J. 2013;19:419–26.
Paluch-Shimon S, Wolf I, Goldberg Evron E, Papa MZ, Shabtai M, et al. High efficacy of pre-operative trastuzumab combined with paclitaxel following doxorubicin & cyclophosphamide in operable breast cancer. Acta Oncol. 2008;47:1564–9.
Von Minckwitz G, Untch M, Nuesch E, Schot M, van Werkhoven E, Wesseling J, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125:145–56.
Von Minckwitz G, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J Clin Oncol. 2010;28:2015–23.
Untch M, Fasching PA, Konecny GE, Fasching PA, Huober J, Tesch H, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2–overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–7.
Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13:135–44.
Pierga JY, Delaloge S, Espié M, Brain E, Sigal-Zafrani B, Mathieu MC, et al. A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat. 2010;122:429–37.
Tanaka S, Iwamoto M, Kimura K, Matsunami N, Morishima H, Yoshidome K, et al. Phase II study of neoadjuvant anthracycline-based regimens combined with nanoparticle albumin-bound paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive operable breast cancer. Clin Breast Cancer. 2015;15:191–6.
Shimizu T, Hirano A, Kamimura M, Ogura K, Kim N, Watanabe O, et al. A phase II study of epirubicin and cyclophosphamide followed by weekly paclitaxel with or without trastuzumab as primary systemic therapy in locally advanced breast cancer. Anticancer Res. 2010;30:4665–71.
Acknowledgments
We thank Tsuneo Yamashiro for his help in the statistical design of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Okamoto, S., Yamada, T., Kanemaki, Y. et al. Magnetic resonance examination to predict pathological complete response following neoadjuvant chemotherapy: when is it appropriate for HER2-positive and triple-negative breast cancers?. Breast Cancer 23, 789–796 (2016). https://doi.org/10.1007/s12282-015-0642-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-015-0642-7