Abstract
Purpose
HER2 +- amplified breast cancer patients derive benefit from treatment with anti-HER2-targeted therapy. Though adjuvant treatment is based on final pathology, decisions regarding neoadjuvant chemotherapy are made in the preoperative setting with imaging playing a key role in staging. We examined the accuracy of pre-operative imaging in determining pathological tumor size (pT) in patients undergoing upfront surgery.
Methods
Early (cT1–T2N0) HER2 + breast cancer patients who underwent upfront surgery between 2015 and 2016 were identified from a prospective institutional database. We compared data for both clinical and final pathologic stage. Only those who underwent magnetic resonance imaging (MRI), mammography, and ultrasound in the preoperative setting were included in the analysis. Adjuvant treatment regimens were reviewed.
Results
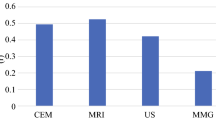
We identified 87 cT1–2N0 patients with invasive HER2 + breast cancer who underwent upfront surgery. Median age was 52 years (IQR 43, 58) and median tumor size was 1.1 cm (IQR 0.5, 1.6). Fifteen patients (17%) were upstaged to stage II/III based on final pathology. Thirty-seven patients were T1cN0 on final pathology; 8 were cT1a–bN0 preop and 12 had pT overestimated by MRI by an average of 1.5 cm (> 0.5–1.5 cm). Compared to both mammography and MRI, the imaging modality most predictive of pT was ultrasound (p = 0.000072 ultrasound vs mammography and 0.000042 ultrasound vs MRI).
Conclusion
For small HER2 + cN0 tumors undergoing upfront surgery, ultrasound was the imaging modality most predictive of pT. MRI overestimated tumor size in approximately 40% of patients. MRI may not accurately discriminate low-volume tumor burden in the breast and carries the potential of overtreatment in the upfront setting.


Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182. https://doi.org/10.1126/science.3798106
The National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) Program. National Cancer Institute (2020). https://seer.cancer.gov/statfacts/html/breast-subtypes. Accessed 1 Aug 2020
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. https://doi.org/10.1056/NEJMoa052122
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672. https://doi.org/10.1056/NEJMoa052306
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283. https://doi.org/10.1056/NEJMoa0910383
Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr et al (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32(33):3744–3752. https://doi.org/10.1200/JCO.2014.55.5730
Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM et al (2013) 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382(9897):1021–1028. https://doi.org/10.1016/S0140-6736(13)61094-6
Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E et al (2017) 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389(10075):1195–1205. https://doi.org/10.1016/S0140-6736(16)32616-2
NCCN Clinical Practice Guidelines in Oncology (2020) National Comprehensive Cancer Network (NCCN). https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 1 Aug 2020
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G et al (2017) Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377(2):122–131. https://doi.org/10.1056/NEJMoa1703643
Siu AL, Force USPST (2016) Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 164(4):279–96. https://doi.org/10.7326/M15-2886
Kolb TM, Lichy J, Newhouse JH (1998) Occult cancer in women with dense breasts: detection with screening US–diagnostic yield and tumor characteristics. Radiology 207(1):191–199. https://doi.org/10.1148/radiology.207.1.9530316
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225(1):165–175. https://doi.org/10.1148/radiol.2251011667
Wells C, DeBruhl N (2011) Magnetic resonance imaging: indications and interpretation. In: Bassett L, Mahoney M, Apple S, D’Orsi C (eds) Breast imaging. Sounders, Philadelphia
Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology 292(3):520–536. https://doi.org/10.1148/radiol.2019182947
Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM et al (2008) Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 26(19):3248–3258. https://doi.org/10.1200/JCO.2007.15.2108
Al-Hallaq HA, Mell LK, Bradley JA, Chen LF, Ali AN, Weichselbaum RR et al (2008) Magnetic resonance imaging identifies multifocal and multicentric disease in breast cancer patients who are eligible for partial breast irradiation. Cancer 113(9):2408–2414. https://doi.org/10.1002/cncr.23872
Esserman L, Hylton N, Yassa L, Barclay J, Frankel S, Sickles E (1999) Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol 17(1):110–119. https://doi.org/10.1200/JCO.1999.17.1.110
Drew PJ, Chatterjee S, Turnbull LW, Read J, Carleton PJ, Fox JN et al (1999) Dynamic contrast enhanced magnetic resonance imaging of the breast is superior to triple assessment for the pre-operative detection of multifocal breast cancer. Ann Surg Oncol 6(6):599–603. https://doi.org/10.1007/s10434-999-0599-x
Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A, Simonetti G et al (2004) Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol 183(4):1149–1157. https://doi.org/10.2214/ajr.183.4.1831149
Houssami N, Turner RM, Morrow M (2017) Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res Treat 165(2):273–283. https://doi.org/10.1007/s10549-017-4324-3
Morrow M, Waters J, Morris E (2011) MRI for breast cancer screening, diagnosis, and treatment. Lancet 378(9805):1804–1811. https://doi.org/10.1016/S0140-6736(11)61350-0
Houssami N, Hayes DF (2009) Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin 59(5):290–302. https://doi.org/10.3322/caac.20028
Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T et al (2011) Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET—randomised controlled trial. Eur J Cancer 47(6):879–886. https://doi.org/10.1016/j.ejca.2010.11.035
Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V et al (2010) Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 375(9714):563–571. https://doi.org/10.1016/S0140-6736(09)62070-5
Houssami N, Turner R, Macaskill P, Turnbull LW, McCready DR, Tuttle TM et al (2014) An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol 32(5):392–401. https://doi.org/10.1200/JCO.2013.52.7515
Balic M, Thomssen C, Wurstlein R, Gnant M, Harbeck N (2019) St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care (Basel) 14(2):103–110. https://doi.org/10.1159/000499931
Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M et al (2016) How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol 23(11):3467–3474. https://doi.org/10.1245/s10434-016-5246-8
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline focused update. J Clin Oncol 36(20):2105–2122. https://doi.org/10.1200/JCO.2018.77.8738
Jethava A, Ali S, Wakefield D, Crowell R, Sporn J, Vrendenburgh J (2015) Diagnostic accuracy of MRI in predicting breast tumor size: comparative analysis of MRI vs histopathological assessed breast tumor size. Conn Med 79(5):261–267
Yoo EY, Nam SY, Choi HY, Hong MJ (2018) Agreement between MRI and pathologic analyses for determination of tumor size and correlation with immunohistochemical factors of invasive breast carcinoma. Acta Radiol 59(1):50–57. https://doi.org/10.1177/0284185117705010
Stuart A (1955) A test for homogeneity of the marginal distributions in a two-way classification. Biometrika 42(3/4):412–416
Maxwell AE (1970) Comparing the classification of subjects by two independent judges. Br J Psychiatry 116(535):651–655. https://doi.org/10.1192/bjp.116.535.651
Wasif N, Garreau J, Terando A, Kirsch D, Mund DF, Giuliano AE (2009) MRI versus ultrasonography and mammography for preoperative assessment of breast cancer. Am Surg 75(10):970–975
Davis PL, Staiger MJ, Harris KB, Ganott MA, Klementaviciene J, McCarty KS Jr et al (1996) Breast cancer measurements with magnetic resonance imaging, ultrasonography, and mammography. Breast Cancer Res Treat 37(1):1–9. https://doi.org/10.1007/BF01806626
Boetes C, Mus RD, Holland R, Barentsz JO, Strijk SP, Wobbes T et al (1995) Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology 197(3):743–747. https://doi.org/10.1148/radiology.197.3.7480749
Gruber IV, Rueckert M, Kagan KO, Staebler A, Siegmann KC, Hartkopf A et al (2013) Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer 13:328. https://doi.org/10.1186/1471-2407-13-328
Katz B, Raker C, Edmonson D, Gass J, Stuckey A, Rizack T (2017) Predicting breast tumor size for pre-operative planning: which imaging modality is best? Breast J 23(1):52–58. https://doi.org/10.1111/tbj.12680
Caramella T, Chapellier C, Ettore F, Raoust I, Chamorey E, Balu-Maestro C (2007) Value of MRI in the surgical planning of invasive lobular breast carcinoma: a prospective and a retrospective study of 57 cases: comparison with physical exam, conventional imaging, and histology. Clin Imag 31:155–161. https://doi.org/10.1016/j.clinimag.2007.01.001
Wasif N, Garreau J, Terando A, Kirsch D, Mund DF, Giuliano AE (2009) MRI versus ultrasonography and mammography for preoperative assessment of breast cancer. Am Surg 75:970–975
Bosch AM, Kessels AG, Beets GL, Rupa JD, Koster D, van Engelshoven JM et al (2003) Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: a prospective study on 105 invasive tumours. Eur J Radiol 48:285–292. https://doi.org/10.1016/s0720-048x(03)00081-0
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK et al (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2):134–141. https://doi.org/10.1056/NEJMoa1406281
Tolaney SM, Guo H, Pernas S, Barry WT, Dillon DA, Ritterhouse L et al (2019) Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 37(22):1868–1875. https://doi.org/10.1200/JCO.19.00066
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380(7):617–628. https://doi.org/10.1056/NEJMoa1814017
DAPHNe: Paclitaxel/Trastuzumab/Pertuzumab in HER2-Positive BC (2018) Dana-Farber Cancer Institute. https://clinicaltrials.gov/ct2/show/NCT03716180. Accessed 1 Sept 2020
Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES et al (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. https://doi.org/10.1200/JCO.20.03399
Haraldsdottir KH, Jonsson T, Halldorsdottir AB, Tranberg KG, Asgeirsson KS (2017) Tumor size of invasive breast cancer on magnetic resonance imaging and conventional imaging (mammogram/ultrasound): comparison with pathological size and clinical implications. Scand J Surg 106(1):68–73. https://doi.org/10.1177/1457496916631855
Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F et al (2009) High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol 27(34):5700–5706. https://doi.org/10.1200/jco.2009.23.2025
Acknowledgements
Jessica Moore, Shan-san Wu, Tiana Le, Summer Koop, and Jessica Massler from Memorial Sloan Kettering Cancer Center assisted with editing and submission. Emanuela Ferraro is funded by the American–Italian Cancer Foundation Post-Doctoral Fellowship.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Contributions
AB, EF, and VS conceptualized and designed the project and performed the interpretation. VS performed the data analysis. TL performed the data acquisition and HH and SJ verified the data. AB drafted the initial manuscript. AB, EF, MM, and MF assisted in the critical revision of the manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Memorial Sloan Kettering Cancer Center approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Botty van den Bruele, A., Ferraro, E., Sevilimedu, V. et al. Does preoperative MRI accurately stratify early-stage HER2 + breast cancer patients to upfront surgery vs neoadjuvant chemotherapy?. Breast Cancer Res Treat 189, 307–315 (2021). https://doi.org/10.1007/s10549-021-06331-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06331-3