Abstract
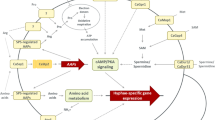
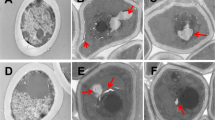
Candida albicans is amajor fungal pathogen in humans. Antimicrobial peptides (AMPs) are critical components of the innate immune response in vertebrates and represent the first line of defense against microbial infection. LL-37 is the only member of the human family of cathelicidin AMPs and is commonly expressed by various tissues and cells, including surfaces of epithelia. The candidacidal effects of LL-37 have been well documented, but the mechanisms by which LL-37 kills C. albicans are not completely understood. In this study, we examined the effects of LL-37 on cell wall and cellular responses in C. albicans. Using transmission electron microscopy, carbohydrate analyses, and staining for β-1,3-glucan, changing of C. albicans cell wall integrity was detected upon LL-37 treatment. In addition, LL-37 also affected cell wall architecture of the pathogen. Finally, DNA microarray analysis and quantitative PCR demonstrated that sub-lethal concentrations of LL-37 modulated the expression of genes with a variety of functions, including transporters, regulators for biological processes, response to stress or chemical stimulus, and pathogenesis. Together, LL-37 induces complex responses in C. albicans, making LL-37 a promising candidate for use as a therapeutic agent against fungal infections.
Similar content being viewed by others
Refrerences
Adams, D.J. 2004. Fungal cell wall chitinases and glucanases. Microbiology 150, 2029–2035.
Bergsson, G., Reeves, E.P., McNally, P., Chotirmall, S.H., Greene, C.M., Greally, P., Murphy, P., O’Neill, S.J., and McElvaney, N.G. 2009. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J. Immunol. 183, 543–551.
Bowdish, D.M., Davidson, D.J., Lau, Y.E., Lee, K., Scott, M.G., and Hancock, R.E. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77, 451–459.
Boxx, G.M., Kozel, T.R., Nishiya, C.T., and Zhang, M.X. 2010. Influence of mannan and glucan on complement activation and C3 binding by Candida albicans. Infect. Immun. 78, 1250–1259.
Braun, B.R., Kadosh, D., and Johnson, A.D. 2001. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. Embo. J. 20, 4753–4761.
Brogden, K.A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250.
Bulik, D.A., Olczak, M., Lucero, H.A., Osmond, B.C., Robbins, P.W., and Specht, C.A. 2003. Chitin synthesis in Saccharomyces cerevisiae in response to supplementation of growth medium with glucosamine and cell wall stress. Eukaryot. Cell 2, 886–900.
Burton, M.F. and Steel, P.G. 2009. The chemistry and biology of LL-37. Nat. Prod. Rep. 26, 1572–1584.
Chang, H.T., Tsai, P.W., Huang, H.H., Liu, Y.S., Chien, T.S., and Lan, C.Y. 2012. LL37 and hBD-3 elevate the beta-1,3-exoglucanase activity of Candida albicans Xog1p, resulting in reduced fungal adhesion to plastic. Biochem. J. 441, 963–970.
den Hertog, A.L., van Marle, J., van Veen, H.A., Van’t Hof, W., Bolscher, J.G., Veerman, E.C., and Nieuw Amerongen, A.V. 2005. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 388, 689–695.
den Hertog, A.L., van Marle, J., Veerman, E.C.I., Valentijn-Benz, M., Nazmi, K., Kalay, H., Grun, C.H., van’t Hof, W., Bolscher, J.G.M., and Amerongen, A.V.N. 2006. The human cathelicidin peptide LL-37 and truncated variants induce segregation of lipids and proteins in the plasma membrane of Candida albicans. Biol. Chem. 387, 1495–1502.
Desai, J.V., Bruno, V.M., Ganguly, S., Stamper, R.J., Mitchell, K.F., Solis, N., Hill, E.M., Xu, W., Filler, S.G., Andes, D.R., and et al. 2013. Regulatory role of glycerol in Candida albicans biofilm formation. mBio 4, e00637-0062.
Dorschner, R.A., Pestonjamasp, V.K., Tamakuwala, S., Ohtake, T., Rudisill, J., Nizet, V., Agerberth, B., Gudmundsson, G.H., and Galloet, R.L. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 117, 91–97.
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356.
Edmond, M.B., Wallace, S.E., McClish, D.K., Pfaller, M.A., Jones, R.N., and Wenzel, R.P. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29, 239–244.
Eggimann, P., Garbino, J., and Pittet, D. 2003. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 3, 685–702.
Finkel, J.S., Xu, W., Huang, D., Hill, E.M., Desai, J.V., Woolford, C.A., Nett, J.E., Taff, H., Norice, C.T., Andes, D.R., and et al. 2012. Portrait of Candida albicans adherence regulators. PLoS Pathog. 8, e100225.
Fonzi, W.A. 1999. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of beta- 1,3- and beta-1,6-glucans. J. Bacteriol. 181, 7070–7079.
Francois, J.M. 2006. A simple method for quantitative determination of polysaccharides in fungal cell walls. Nat. Protoc. 1, 2995–3000.
Galan-Diez, M., Arana, D.M., Serrano-Gomez, D., Kremer, L., Casasnovas, J.M., Ortega, M., Cuesta-Dominguez, A., Corbi, A.L., Pla, J., and Fernandez-Ruiz, E. 2010. Candida albicans betaglucan exposure is controlled by the fungal CEK1-mediated mitogen- activated protein kinase pathway that modulates immune responses triggered through dectin-1. Infect. Immun. 78, 1426–1416.
Gonzalez, M.M., Diez-Orejas, R., Molero, G., Alvarez, A.M., Pla, J., Nombela, C., and Sanchez-Perez, M. 1997. Phenotypic characterization of a Candida albicans strain deficient in its major exoglucanase. Microbiology 143(Pt 9), 3023–3032.
Henzler Wildman, K.A., Lee, D.K., and Ramamoorthy, A. 2003. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 42, 6545–6558.
Hsu, P.C., Chao, C.C., Yang, C.Y., Ye, Y.L., Liu, F.C., Chuang, Y.J., and Lan, C.Y. 2013. Diverse Hap43-independent functions of the Candida albicans CCAAT-binding complex. Eukaryot. Cell 12, 804–815.
Hurtado-Guerrero, R., Schuttelkopf, A.W., Mouyna, I., Ibrahim, A.F., Shepherd, S., Fontaine, T., Latge, J.P., and van Aalten, D.M. 2009. Molecular mechanisms of yeast cell wall glucan remodeling. J. Biol. Chem. 284, 8461–8469.
Jenssen, H., Hamill, P., and Hancock, R.E. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19, 491–511.
Johansson, J., Gudmundsson, G.H., Rottenberg, M.E., Berndt, K.D., and Agerberth, B. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273, 3718–3724.
Kai-Larsen, Y. and Agerberth, B. 2008. The role of the multifunctional peptide LL-37 in host defense. Front Biosci. 13, 3760–3767.
Klis, F.M., de Groot, P., and Hellingwerf, K. 2001. Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39 Suppl 1, 1–8.
Kollar, R., Reinhold, B.B., Petrakova, E., Yeh, H.J., Ashwell, G., Drgonova, J., Kapteyn, J.C., Klis, F.M., and Cabib, E. 1997. Architecture of the yeast cell wall. Beta(1→6)-glucan interconnects mannoprotein, beta(1→)3-glucan, and chitin. J. Biol. Chem. 272, 17762–17775.
Kumar, R., Chadha, S., Saraswat, D., Bajwa, J.S., Li, R.A., Conti, H.R., and Edgerton, M. 2011. Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins. J. Biol. Chem. 286, 43748–43758.
Lesage, G. and Bussey, H. 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 317–343.
Li, R., Kumar, R., Tati, S., Puri, S., and Edgerton, M. 2013. Candida albicans flu1-mediated efflux of salivary histatin 5 reduces its cytosolic concentration and fungicidal activity. Antimicrob. Agents Chemother. 57, 1832–1839.
Lopez-Garcia, B., Lee, P.H.A., Yamasaki, K., and Gallo, R.L. 2005. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J. Invest. Dermatol. 125, 108–115.
Luo, L., Tong, X., and Farley, P.C. 2007. The Candida albicans gene HGT12 (orf19.7094) encodes a hexose transporter. FEMS Immunol. Medical Microbiol. 51, 14–17.
Martinez-Esparza, M., Sarazin, A., Jouy, N., Poulain, D., and Jouault, T. 2006. Comparative analysis of cell wall surface glycan expression in Candida albicans and Saccharomyces cerevisiae yeasts by flow cytometry. J. Immunol. Methods 314, 90–102.
Masuoka, J. 2004. Surface glycans of Candida albicans and other pathogenic fungi: physiological roles, clinical uses, and experimental challenges. Clin. Microbiol. Rev. 17, 281–310.
Mio, T., Yamada-Okabe, T., Yabe, T., Nakajima, T., Arisawa, M., and Yamada-Okabe, H. 1997. Isolation of the Candida albicans homologs of Saccharomyces cerevisiae KRE6 and SKN1: expression and physiological function. J. Bacteriol. 179, 2363–2372.
Murad, A.M., Leng, P., Straffon, M., Wishart, J., Macaskill, S., MacCallum, D., Schnell, N., Talibi, D., Marechal, D., Tekaia, F., and et al. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. Embo. J. 20, 4742–4752.
Nather, K. and Munro, C.A. 2008. Generating cell surface diversity in Candida albicans and other fungal pathogens. FEMS Microbiol. Lett. 285, 137–145.
Nijnik, A. and Hancock, R.E. 2009. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 16, 41–47.
Nobile, C.J., Fox, E.P., Nett, J.E., Sorrells, T.R., Mitrovich, Q.M., Hernday, A.D., Tuch, B.B., Andes, D.R., and Johnson, A.D. 2012. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148, 126–138.
Overhage, J., Campisano, A., Bains, M., Torfs, E.C., Rehm, B.H., and Hancock, R.E. 2008. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 76, 4176–4182.
Pfaller, M.A. and Diekema, D.J. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163.
Pietiainen, M., Gardemeister, M., Mecklin, M., Leskela, S., Sarvas, M., and Kontinen, V.P. 2005. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 151, 1577–1592.
Reddy, K.V., Yedery, R.D., and Aranha, C. 2004. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents 24, 536–547.
Ruiz-Herrera, J., Elorza, M.V., Valentin, E., and Sentandreu, R. 2006. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 6, 14–29.
Scouten, W.H. 1984. Methods of enzymatic analysis, 3rd Edition, Vol. 1, Fundamentals-Bergmeyer, Hu. J. Am. Chem. Soc. 106, 1535–1535.
Senyurek, I., Paulmann, M., Sinnberg, T., Kalbacher, H., Deeg, M., Gutsmann, T., Hermes, M., Kohler, T., Gotz, F., Wolz, C., and et al. 2009. Dermcidin-derived peptides show a different mode of action than the cathelicidin LL-37 against Staphylococcus aureus. Antimicrob. Agents Chemother. 53, 2499–2509.
Sorensen, O.E., Follin, P., Johnsen, A.H., Calafat, J., Tjabringa, G.S., Hiemstra, P.S., and Borregaard, N. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959.
Tsai, P.W., Yang, C.Y., Chang, H.T., and Lan, C.Y. 2011a. Characterizing the role of cell-wall beta-1,3-exoglucanase Xog1p in Candida albicans adhesion by the human antimicrobial peptide LL-37. PLoS ONE 6, e21394.
Tsai, P.W., Yang, C.Y., Chang, H.T., and Lan, C.Y. 2011b. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 6, e17755.
Turner, J., Cho, Y., Dinh, N.N., Waring, A.J., and Lehrer, R.I. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42, 2206–2214.
Vyas, V.K., Berkey, C.D., Miyao, T., and Carlson, M. 2005. Repressors Nrg1 and Nrg2 regulate a set of stress-responsive genes in Saccharomyces cerevisiae. Eukaryot. Cell. 4, 1882–1891.
Vylkova, S., Jang, W.S., Li, W., Nayyar, N., and Edgerton, M. 2007. Histatin 5 initiates osmotic stress response in Candida albicans via activation of the Hog1 mitogen-activated protein kinase pathway. Eukaryot. Cell. 6, 1876–1888.
Wheeler, R.T. and Fink, G.R. 2006. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2, e35.
Wisplinghoff, H., Bischoff, T., Tallent, S.M., Seifert, H., Wenzel, R.P., and Edmond, M.B. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39, 309–317.
Wunder, D., Dong, J., Baev, D., and Edgerton, M. 2004. Human salivary histatin 5 fungicidal action does not induce programmed cell death pathways in Candida albicans. Antimicrob. Agents Chemother. 48, 110–115.
Yeaman, M.R. and Yount, N.Y. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55, 27–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, PW., Cheng, YL., Hsieh, WP. et al. Responses of Candida albicans to the human antimicrobial peptide LL-37. J Microbiol. 52, 581–589 (2014). https://doi.org/10.1007/s12275-014-3630-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-014-3630-2