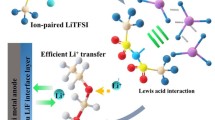
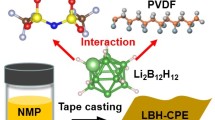
Abstract
Polymer-based solid electrolytes have been extensively studied for solid-state lithium metal batteries to achieve high energy density and reliable security. But, its practical application is severely limited by low ionic conductivity and slow Li+ transference. Herein, based on the “binary electrolytes” of poly(vinylidene fluoride-chlorotrifluoroethylene) (P(VDF-CTFE)) and lithium salt (LiTFSI), a kind of eutectogel hybrid electrolytes (EHEs) with high Li+ transference number was developed via tuning the spontaneous coupling of charge and vacated space generated by Li-cation diffusion utilizing the Li6.4La3Zr1.4Ta0.6O12 (LLZTO) dopant. LLZTO doping promotes the dissociation of lithium salt, increases Li+ carrier density, and boosts ion jumping and the coordination/decoupling reactions of Li+. As a result, the optimized EHEs-10% possess a high Li-transference number of 0.86 and a high Li+ conductivity of 3.2×10−4 S·cm−1 at room temperature. Moreover, the prepared EHEs-10% composite solid electrolyte presents excellent lithiumphilic and compatibility, and can be tested stably for 1,200 h at 0.3 mA·cm−2 with assembled lithium symmetric batteries. Likewise, the EHEs-10% films match well with high-loading LiFePO4 and LiCoO2 cathodes (> 10 mg·cm−2) and exhibit remarkable interface stability. Particularly, the LiFePO4//EHEs-10%//Li and LiCoO2//EHEs-10%//Li cells deliver high rate performance of 118 mA·hg−1 at 1 C and 93.7 mAh·g−1 at 2 C with coulombic efficiency of 99.3% and 98.1%, respectively. This work provides an in-depth understanding and new insights into our design for polymer electrolytes with fast Li+ diffusion.

Similar content being viewed by others
References
Zhao, Y.; Wang, L.; Zhou, Y. N.; Liang, Z.; Tavajohi, N.; Li, B. H.; Li, T. Solid polymer electrolytes with high conductivity and transference number of Li ions for Li-based rechargeable batteries. Adv. Sci. 2021, 8, 2003675.
Wang, J. R.; Li, S. Q.; Zhao, Q.; Song, C.; Xue, Z. G. Structure code for advanced polymer electrolyte in lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2008208.
Wang, S. Z.; Wang, Y.; Song, Y. C.; Jia, X. H.; Yang, J.; Li, Y.; Liao, J. X.; Song, H. J. Immobilizing polysulfide via multiple active sites in W18O49 for Li-S batteries by oxygen vacancy engineering. Energy Storage Mater. 2021, 43, 422–429.
Wang, S. Z.; Liao, J. X.; Yang, X. F.; Liang, J. N.; Sun, Q.; Liang, J. W.; Zhao, F. P.; Koo, A.; Kong, F. P.; Yao, Y. et al. Designing a highly efficient polysulfide conversion catalyst with paramontroseite for high-performance and long-life lithium-sulfur batteries. Nano Energy 2019, 57, 230–240.
Wang, S. Z.; Feng, S. P.; Liang, J. W.; Su, Q. M.; Zhao, F. P.; Song, H. J.; Zheng, M.; Sun, Q.; Song, Z. X.; Jia, X. H. et al. Insight into MoS2-MoN heterostructure to accelerate polysulfide conversion toward high-energy-density lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2003314.
Lu, L. J.; Ding, W. Q.; Liu, J. Q.; Yang, B. Flexible PVDF based piezoelectric nanogenerators. Nano Energy 2020, 78, 105251.
Sun, N.; Liu, Q. S.; Cao, Y.; Lou, S. F.; Ge, M. Y.; Xiao, X. H.; Lee, W. K.; Gao, Y. Z.; Yin, G. P.; Wang, J. J. et al. Anisotropically electrochemical-mechanical evolution in solid-state batteries and interfacial tailored strategy. Angew. Chem., Int. Ed. 2019, 58, 18647–18653.
Wang, L. G.; Dai, A.; Xu, W. Q.; Lee, S.; Cha, W.; Harder, R.; Liu, T. C.; Ren, Y.; Yin, G. P.; Zuo, P. J. et al. Structural distortion induced by manganese activation in a lithium-rich layered cathode. J. Am. Chem. Soc. 2020, 142, 14966–14973.
Yu, X. W.; Manthiram, A. A review of composite polymer-ceramic electrolytes for lithium batteries. Energy Storage Mater. 2021, 34, 282–300.
Choo, Y.; Halat, D. M.; Villaluenga, I.; Timachova, K.; Balsara, N. P. Diffusion and migration in polymer electrolytes. Prog. Polym. Sci. 2020, 103, 101220.
Lou, S. F.; Liu, Q. W.; Zhang, F.; Liu, Q. S.; Yu, Z. J.; Mu, T. S.; Zhao, Y.; Borovilas, J.; Chen, Y. J.; Ge, M. Y. et al. Insights into interfacial effect and local lithium-ion transport in polycrystalline cathodes of solid-state batteries. Nat. Commun. 2020, 11, 5700.
Wang, S. Z.; Wang, Y.; Song, Y. C.; Zhang, J. T.; Jia, X. H.; Yang, J.; Shao, D.; Li, Y.; Liao, J. X.; Song, H. J. Synergistic regulating of dynamic trajectory and lithiophilic nucleation by Heusler alloy for dendrite-free Li deposition. Energy Storage Mater. 2022, 50, 505–513.
Tang, S.; Guo, W.; Fu, Y. Z. Advances in composite polymer electrolytes for lithium batteries and beyond. Adv. Energy Mater. 2021, 11, 2000802.
Zhang, F.; Lou, S. F.; Li, S.; Yu, Z. J.; Liu, Q. S.; Dai, A.; Cao, C. T.; Toney, M. F.; Ge, M. Y.; Xiao, X. H. et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 2020, 11, 3050.
Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. Energy 2022, DOI: https://doi.org/10.26599/NRE.2022.9120016.
Doyle, M.; Fuller, T. F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533.
Lou, S. F.; Yu, Z. J.; Liu, Q. S.; Wang, H.; Chen, M.; Wang, J. J. Multi-scale imaging of solid-state battery interfaces: From atomic scale to macroscopic scale. Chem 2020, 6, 2199–2218.
Wu, Y. X.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q. G.; Chen, M. H. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84.
Chen, G. H.; Zhang, F.; Zhou, Z. M.; Li, J. R.; Tang, Y. B. A flexible dual-ion battery based on PVDF-HFP-modified gel polymer electrolyte with excellent cycling performance and superior rate capability. Adv. Energy Mater. 2018, 8, 1801219.
Wang, L. J.; Wang, Z. H.; Sun, Y.; Liang, X.; Xiang, H. F. Sb2O3 modified PVDF-CTFE electrospun fibrous membrane as a safe lithium-ion battery separator. J. Membr. Sci. 2019, 572, 512–519.
Liu, Q.; Geng, Z.; Han, C. P.; Fu, Y. Z.; Li, S.; He, Y. B.; Kang, F. Y.; Li, B. H. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J. Power Sources 2018, 389, 120–134.
Zhao, N.; Khokhar, W.; Bi, Z. J.; Shi, C.; Guo, X. X.; Fan, L. Z.; Nan, C. W. Solid garnet batteries. Joule 2019, 3, 1190–1199.
Chen, S. J.; Zhang, J. X.; Nie, L.; Hu, X. C.; Huang, Y. Q.; Yu, Y.; Liu, W. All-solid-state batteries with a limited lithium metal anode at room temperature using a garnet-based electrolyte. Adv. Mater. 2021, 33, 2002325.
Huo, H. Y.; Luo, J.; Thangadurai, V.; Guo, X. X.; Nan, C. W.; Sun, X. L. Li2CO3: A critical issue for developing solid garnet batteries. ACS Energy Lett. 2020, 5, 252–262.
Ma, C.; Rangasamy, E.; Liang, C. D.; Sakamoto, J.; More, K. L.; Chi, M. F. Excellent stability of a lithium-ion-conducting solid electrolyte upon reversible Li+/H+ exchange in aqueous solutions. Angew. Chem., Int. Ed. 2015, 54, 129–133.
Truong, L.; Thangadurai, V. Soft-chemistry of garnet-type Li5+xBaxLa3−xNb2O12 (x = 0, 0.5, 1): Reversible H+ ↔ Li+ ionexchange reaction and their X-ray, 7Li MAS NMR, IR, and AC impedance spectroscopy characterization. Chem. Mater. 2011, 23, 3970–3977.
Lou, S. F.; Zhang, F.; Fu, C. K.; Chen, M.; Ma, Y. L.; Yin, G. P.; Wang, J. J. Interface issues and challenges in all-solid-state batteries: Lithium, sodium, and beyond. Adv. Mater. 2021, 33, 2000721.
Sharafi, A.; Yu, S.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H. M.; Nanda, J.; Chi, M. F.; Siegel, D. J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li-Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 2017, 5, 13475–13487.
Wang, Z. Q.; Li, X. Y.; Chen, Y. M.; Pei, K.; Mai, Y. W.; Zhang, S. L.; Li, J. Creep-enabled 3D solid-state lithium-metal battery. Chem 2020, 6, 2878–2892.
Li, R. G.; Wu, D. B.; Yu, L.; Mei, Y. N.; Wang, L. B.; Li, H.; Hu, X. L. Unitized configuration design of thermally stable composite polymer electrolyte for lithium batteries capable of working over a wide range of temperatures. Adv. Eng. Mater. 2019, 21, 1900055.
Zhang, X.; Liu, T.; Zhang, S. F.; Huang, X.; Xu, B. Q.; Lin, Y. H.; Xu, B.; Li, L. L.; Nan, C. W.; Shen, Y. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. A. Am. Chem. Soc. 2017, 139, 13779–13785.
Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem., Int. Ed. 2007, 46, 7778–7781.
Shen, X.; Zhang, Q.; Ning, T.; Liu, T.; Luo, Y.; He, X.; Luo, Z.; Lu, A. Critical challenges and progress of solid garnet electrolytes for all-solid-state batteries. Mater. Today Chem. 2020, 18, 100368.
Huo, H. Y.; Chen, Y.; Li, R. Y.; Zhao, N.; Luo, J.; da Silva, J. G. P.; Mücke, R.; Kaghazchi, P.; Guo, X. X.; Sun, X. L. Design of a mixed conductive garnet/Li interface for dendrite-free solid lithium metal batteries. Energy Environ. Sci. 2020, 13, 127–134.
Zha, W. P.; Chen, F.; Yang, D. J.; Shen, Q.; Zhang, L. M. Highperformance Li64La3Zr1.4Ta0.6O12/poly(ethylene oxide)/Succinonitrile composite electrolyte for solid-state lithium batteries. J. Power Sources 2018, 397, 87–94.
Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727.
Diederichsen, K. M.; McShane, E. J.; McCloskey, B. D. Promising routes to a high Li+ transference number electrolyte for lithium ion batteries. ACS Energy Lett. 2017, 2, 2563–2575.
Yadav, P. J. P.; Maiti, B.; Ghorai, B. K.; Sastry, P. U.; Patra, A. K.; Aswal, V. K.; Maiti, P. Thermoreversible gelation of poly(vinylidene fluoride-co-chlorotrifluoroethylene): Structure, morphology, thermodynamics, and theoretical prediction. Macromolecules 2011, 44, 3029–3038.
Zheng, L. B.; Wang, J.; Yu, D. W.; Zhang, Y.; Wei, Y. S. Preparation of PVDF-CTFE hydrophobic membrane by non-solvent induced phase inversion: Relation between polymorphism and phase inversion. J. Membr. Sci. 2018, 550, 480–491.
Zheng, L. B.; Wang, J.; Wu, Z. J.; Li, J.; Zhang, Y.; Yang, M.; Wei, Y. S. Preparation of interconnected biomimetic poly(vinylidene fluoride-co-chlorotrifluoroethylene) hydrophobic membrane by tuning the two-stage phase inversion process. ACS Appl. Mater. Interfaces 2016, 8, 32604–32615.
Huang, Y. F.; Xu, J. Z.; Soulestin, T.; Dos Santos, F. D.; Li, R. P.; Fukuto, M.; Lei, J.; Zhong, G. J.; Li, Z. M.; Li, Y. et al. Can relaxor ferroelectric behavior Be realized for Poly(vinylidene fluoride-co-chlorotrifluoroethylene) [P(VDF-CTFE)] random copolymers by inclusion of CTFE units in PVDF crystals? Macromolecules 2018, 51, 5460–5472.
Salimi, A.; Yousefi, A. A. FTIR studies of β-phase crystal formation in stretched PVDF films. Polym. Test. 2003, 22, 699–704.
Cai, X. M.; Lei, T. P.; Sun, D. H.; Lin, L. W. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389.
Zhang, X.; Han, J.; Niu, X. F.; Xin, C. Z.; Xue, C. J.; Wang, S.; Shen, Y.; Zhang, L.; Li, L. L.; Nan, C. W. High cycling stability for solid-state Li metal batteries via regulating solvation effect in poly(vinylidene fluoride)-based electrolytes. Batter Supercaps 2020, 3, 876–883.
Ma, R. H.; Lu, X. L.; Kong, X.; Zheng, S. Y.; Zhang, S. Z.; Liu, S. H. A method of controllable positive-charged modification of PVDF-CTFE membrane surface based on C-Cl active site. J. Membr. Sci. 2021, 620, 118936.
Zhang, S. Z.; Liang, T. B.; Wang, D. H.; Xu, Y. J.; Cui, Y. L.; Li, J. R.; Wang, X. L.; Xia, X. H.; Gu, C. D.; Tu, J. P. A stretchable and safe polymer electrolyte with a protecting-layer strategy for solidstate lithium metal batteries. Adv. Sci. 2021, 8, 2003241.
Xu, B. Y.; Li, X. Y.; Yang, C.; Li, Y. T.; Grundish, N. S.; Chien, P. H.; Dong, K.; Manke, I.; Fang, R. Y.; Wu, N. et al. Interfacial chemistry enables stable cycling of all-solid-state Li metal batteries at high current densities. J. Am. Chem. Soc. 2021, 143, 6542–6550.
Xue, C. J.; Guan, S. D.; Hu, B. K.; Wang, X. Z.; Xin, C. Z.; Liu, S. J.; Yu, J. Y.; Wen, K. H.; Li, L. L.; Nan, C. W. Significantly improved interface between PVDF-based polymer electrolyte and lithium metal via thermal-electrochemical treatment. Energy Storage Mater. 2022, 46, 452–460.
Acknowledgements
This work was supported by the International Cooperation Projects of Sichuan Provincial Department of Science and Technology (No. 2021YFH0126) and Quzhou Science and Technology Bureau Project (No. 2021D006), and the Fundamental Research Funds for the Central Universities (No. A030202063008029). The China Postdoctoral Science Foundation (Nos. 2021T140433, 2020M683408) and the Natural Science Foundation of Shaanxi Province (No. 2021JQ-538). The authors would like to thank Qian Fu from shiyanjia Lab (https://www.shiyanjia.com) for support of XPS analysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4759_MOESM1_ESM.pdf
A highly ionic transference number eutectogel hybrid electrolytes based on spontaneous coupling inhibitor for solid-state lithium metal batteries
Rights and permissions
About this article
Cite this article
Bi, L., Wei, X., Qiu, Y. et al. A highly ionic transference number eutectogel hybrid electrolytes based on spontaneous coupling inhibitor for solid-state lithium metal batteries. Nano Res. 16, 1717–1725 (2023). https://doi.org/10.1007/s12274-022-4759-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4759-7