Abstract
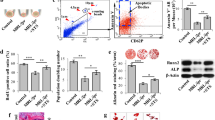
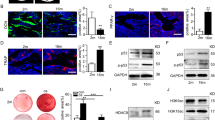
Aging skeletons display decreased bone mass, increased marrow adiposity, and impaired bone marrow mesenchymal stem cells (MSCs). Apoptosis is a programmed cell death process that generates a large number of apoptotic vesicles (apoVs). Dysregulated apoptosis has been closely linked to senescence-associated diseases. However, whether apoVs mediate aging-related bone loss is not clear. In this study, we showed that young MSC-derived apoVs effectively rejuvenated the nuclear abnormalities of aged bone marrow MSCs and restored their impaired self-renewal, osteo-/adipo-genic lineage differentiation capacities via activating autophagy. Mechanistically, apoptotic young MSCs generated and enriched a high level of Ras-related protein 7 (Rab7) into apoVs. Subsequently, recipient aged MSCs reused apoV-derived Rab7 to restore autolysosomes formation, thereby contributing to autophagy flux activation and MSC rejuvenation. Moreover, systemic infusion of young MSC-derived apoVs enhanced bone mass, reduced marrow adiposity, and recused the impairment of recipient MSCs in aged mice. Our findings reveal the role of apoVs in rejuvenating aging-MSCs via restoring autolysosome formation and provide a potential approach for treating age-related bone loss.

Similar content being viewed by others
References
López-Otín, C.; Blasco, M. A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
Hooten, N. N.; Pacheco, N. L.; Smith, J. T.; Evans, M. K. The accelerated aging phenotype: The role of race and social determinants of health on aging. Ageing Res. Rev. 2022, 73, 101536.
He, S. H.; Sharpless, N. E. Senescence in health and disease. Cell 2017, 169, 1000–1011.
Deng, P.; Yuan, Q.; Cheng, Y. D.; Li, J.; Liu, Z. Q.; Liu, Y.; Li, Y.; Su, T.; Wang, J.; Salvo, M. E. et al. Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging. Cell Stem Cell 2021, 28, 1057–1073.e7.
Kawai, M.; Rosen, C. J. Adiposity and bone accrual—Still an established paradigm? Nat. Rev. Endocrinol. 2010, 6, 63–64.
Yamaza, T.; Miura, Y.; Akiyama, K.; Bi, Y. M.; Sonoyama, W.; Gronthos, S.; Chen, W. J.; Le, A.; Shi, S. T. Mesenchymal stem cellmediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood 2009, 113, 2595–2604.
Zaidi, M.; Sun, L.; Blair, H. C. Special stem cells for bone. Cell Stem Cell 2012, 10, 233–234.
Zhang, H. G.; Xu, R. Y.; Li, B.; Xin, Z. L.; Ling, Z. J.; Zhu, W. W.; Li, X.; Zhang, P.; Fu, Y.; Chen, J. Y. et al. LncRNA NEAT1 controls the lineage fates of BMSCs during skeletal aging by impairing mitochondrial function and pluripotency maintenance. Cell Death Differ. 2022, 29, 351–365.
Goodell, M. A.; Rando, T. A. Stem cells and healthy aging. Science 2015, 350, 1199–1204.
Lei, Q.; Gao, F.; Liu, T.; Ren, W. X.; Chen, L.; Cao, Y. L.; Chen, W. L.; Guo, S. J.; Zhang, Q.; Chen, W. Q. et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow-derived mesenchymal stem cells and slow age-related degeneration. Sci. Transl. Med. 2021, 13, eaaz8697.
Somiya, M.; Kuroda, S. Reporter gene assay for membrane fusion of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12171.
Gong, L. Z.; Chen, B.; Zhang, J. T.; Sun, Y. J.; Yuan, J.; Niu, X.; Hu, G. W.; Chen, Y.; Xie, Z. P.; Deng, Z. F. et al. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. J. Extracell. Vesicles 2020, 9, 1800971.
Xiao, X.; Xu, M. Q.; Yu, H. L.; Wang, L. P.; Li, X. X.; Rak, J.; Wang, S. H.; Zhao, R. C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354.
Boulestreau, J.; Maumus, M.; Jorgensen, C.; Noël, D. Extracellular vesicles from mesenchymal stromal cells: Therapeutic perspectives for targeting senescence in osteoarthritis. Adv. Drug Delivery Rev. 2021, 175, 113836.
Tucher, C.; Bode, K.; Schiller, P.; Claßen, L.; Birr, C.; Souto-Carneiro, M. M.; Blank, N.; Lorenz, H. M.; Schiller, M. Extracellular vesicle subtypes released from activated or apoptotic Tlymphocytes carry a specific and stimulus-dependent protein cargo. Front. Immunol. 2018, 9, 534.
Wickman, G. R.; Julian, L.; Mardilovich, K.; Schumacher, S.; Munro, J.; Rath, N.; Zander, S. A.; Mleczak, A.; Sumpton, D.; Morrice, N. et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013, 20, 1293–1305.
Kakarla, R.; Hur, J.; Kim, Y. J.; Kim, J.; Chwae, Y. J. Apoptotic cell-derived exosomes: Messages from dying cells. Exp. Mol. Med. 2020, 52, 1–6.
Zhang, X.; Tang, J. X.; Kou, X. X.; Huang, W. Y.; Zhu, Y.; Jiang, Y. H.; Yang, K. K.; Li, C.; Hao, M.; Qu, Y. et al. Proteomic analysis of MSC-derived apoptotic vesicles identifies Fas inheritance to ameliorate haemophilia A via activating platelet functions. J. Extracell. Vesicles 2022, 11, e12240.
Caruso, S.; Poon, I. K. H. Apoptotic cell-derived extracellular vesicles: More than just debris. Front. Immunol. 2018, 9, 1486.
Wang, J.; Cao, Z. Y.; Wang, P. P.; Zhang, X.; Tang, J. X.; He, Y. F.; Huang, Z. Q.; Mao, X. L.; Shi, S. T.; Kou, X. X. Apoptotic extracellular vesicles ameliorate multiple myeloma by restoring Fasmediated apoptosis. ACS Nano. 2021, 15, 14360–14372.
Zheng, C. X.; Sui, B.; Zhang, X.; Hu, J. C.; Chen, J.; Liu, J.; Wu, D.; Ye, Q. Y.; Xiang, L.; Qiu, X. Y. et al. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J. Extracell. Vesicles 2021, 10, e12109.
Liu, D. W.; Kou, X. X.; Chen, C.; Liu, S. Y.; Liu, Y.; Yu, W. J.; Yu, T. T.; Yang, R. L.; Wang, R. C.; Zhou, Y. H. et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933.
Medina, C. B.; Mehrotra, P.; Arandjelovic, S.; Perry, J. S. A.; Guo, Y. Z.; Morioka, S.; Barron, B.; Walk, S. F.; Ghesquière, B.; Krupnick, A. S. et al. Metabolites released from apoptotic cells act as tissue messengers. Nature 2020, 580, 130–135.
Liu, H.; Liu, S. Y.; Qiu, X. Y.; Yang, X. S.; Bao, L. L.; Pu, F. X.; Liu, X. M.; Li, C. Y.; Xuan, K.; Zhou, J. et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy 2020, 16, 2140–2155.
Argüelles, S.; Guerrero-Castilla, A.; Cano, M.; Muñoz, M. F.; Ayala, A. Advantages and disadvantages of apoptosis in the aging process. Ann. N. Y. Acad. Sci. 2019, 1443, 20–33.
Salminen, A.; Ojala, J.; Kaarniranta, K. Apoptosis and aging: Increased resistance to apoptosis enhances the aging process. Cell. Mol. Life Sci. 2011, 68, 1021–1031.
Leidal, A. M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348.
Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R. I.; Simon, A. K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N. et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650.
Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A. M. Autophagy and the hallmarks of aging. Ageing Res. Rev. 2021, 72, 101468.
Rubinsztein, D. C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695.
Ho, T. T.; Warr, M. R.; Adelman, E. R.; Lansinger, O. M.; Flach, J.; Verovskaya, E. V.; Figueroa, M. E.; Passeguù, E. Autophagy maintains the metabolism and function of young and old stem cells. Nature 2017, 543, 205–210.
Ma, Y.; Qi, M.; An, Y.; Zhang, L. Q.; Yang, R.; Doro, D. H.; Liu, W. J.; Jin, Y. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell 2018, 17, e12709.
Liu, F.; Yuan, Y. J.; Bai, L.; Yuan, L. H.; Li, L.; Liu, J. P.; Chen, Y. N.; Lu, Y. R.; Cheng, J. Q.; Zhang, J. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 2021, 43, 101963.
Mizushima, N.; Levine, B.; Cuervo, A. M.; Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075.
Sasaki, T.; Lian, S. S.; Khan, A.; Llop, J. R.; Samuelson, A. V.; Chen, W. B.; Klionsky, D. J.; Kishi, S. Autolysosome biogenesis and developmental senescence are regulated by both Spns1 and v-ATPase. Autophagy 2017, 13, 386–403.
Ma, L.; Huang, Z. Q.; Wu, D.; Kou, X. X.; Mao, X. L.; Shi, S. T. CD146 controls the quality of clinical grade mesenchymal stem cells from human dental pulp. Stem Cell Res. Ther. 2021, 12, 488.
Huang, R. Q.; Qin, C. J.; Wang, J. M.; Hu, Y. Q.; Zheng, G. P.; Qiu, G. G.; Ge, M. H.; Tao, H. K.; Shu, Q.; Xu, J. G. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging 2019, 11, 7996–8014.
Shi, H. Z.; Zeng, J. C.; Shi, S. H.; Giannakopoulos, H.; Zhang, Q. Z.; Le, A. D. Extracellular vesicles of GMSCs alleviate aging-related cell senescence. J. Dent. Res. 2021, 100, 283–292.
Liu, S. Q.; Mahairaki, V.; Bai, H.; Ding, Z.; Li, J. X.; Witwer, K. W.; Cheng, L. Z. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells 2019, 37, 779–790.
Zhao, D. Y.; Tao, W. H.; Li, S. H.; Chen, Y.; Sun, Y. H.; He, Z. G.; Sun, B. J.; Sun, J. Apoptotic body-mediated intercellular delivery for enhanced drug penetration and whole tumor destruction. Sci. Adv. 2021, 7, eabg0880.
Baar, M. P.; Brandt, R. M. C.; Putavet, D. A.; Klein, J. D. D.; Derks, K. W. J.; Bourgeois, B. R. M.; Stryeck, S.; Rijksen, Y.; Van Willigenburg, H.; Feijtel, D. A. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017, 169, 132–147.e16.
Hu, L.; Li, H. Q.; Zi, M. T.; Li, W.; Liu, J.; Yang, Y.; Zhou, D. H.; Kong, Q. P.; Zhang, Y. X.; He, Y. H. Why senescent cells are resistant to apoptosis: An insight for senolytic development. Front. Cell Dev. Biol. 2022, 10, 822816.
Akers, J. C.; Gonda, D.; Kim, R.; Carter, B. S.; Chen, C. C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro Oncol. 2013, 113, 1–11.
Eitan, E.; Suire, C.; Zhang, S.; Mattson, M. P. Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. 2016, 32, 65–74.
Ansari, M. Y.; Ball, H. C.; Wase, S. J.; Novak, K.; Haqqi, T. M. Lysosomal dysfunction in osteoarthritis and aged cartilage triggers apoptosis in chondrocytes through BAX mediated release of cytochrome c. Osteoarthritis Cartilage 2021, 29, 100–112.
Kitada, M.; Koya, D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021, 17, 647–661.
Shi, B. H.; Ma, M. Q.; Zheng, Y. T.; Pan, Y. Y.; Lin, X. H. mTOR and Beclin1:Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J. Cell. Physiol. 2019, 234, 12562–12568.
Tian, J.; Kou, X. X.; Wang, R. C.; Jing, H.; Chen, C.; Tang, J. X.; Mao, X. L.; Zhao, B. J.; Wei, X.; Shi, S. T. Autophagy controls mesenchymal stem cell therapy in psychological stress colitis mice. Autophagy 2021, 17, 2586–2603.
Chen, W. L.; Xiao, W. W.; Liu, X. Z.; Yuan, P. P.; Zhang, S. Q.; Wang, Y. G.; Wu, W. Pharmacological manipulation of macrophage autophagy effectively rejuvenates the regenerative potential of biodegrading vascular graft in aging body. Bioact. Mater. 2022, 11, 283–299.
Yoshida, G.; Kawabata, T.; Takamatsu, H.; Saita, S.; Nakamura, S.; Nishikawa, K.; Fujiwara, M.; Enokidani, Y.; Yamamuro, T.; Tabata, K. et al. Degradation of the NOTCH intracellular domain by elevated autophagy in osteoblasts promotes osteoblast differentiation and alleviates osteoporosis. Autophagy, in press, https://doi.org/10.1080/15548627.2021.2017587.
Gong, Y.; Li, Z. Q.; Zou, S. T.; Deng, D. Z.; Lai, P. L.; Hu, H. L.; Yao, Y. Z.; Hu, L.; Zhang, S.; Li, K. et al. Vangl2 limits chaperone-mediated autophagy to balance osteogenic differentiation in mesenchymal stem cells. Dev. Cell 2021, 56, 2103–2120.e9.
Li, X.; Xu, J. K.; Dai, B. Y.; Wang, X. L.; Guo, Q. Y.; Qin, L. Targeting autophagy in osteoporosis: From pathophysiology to potential therapy. Ageing Res. Rev. 2020, 62, 101098.
Zhao, Y. G.; Codogno, P.; Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 2021, 22, 733–750.
Sasaki, T.; Lian, S. S.; Qi, J.; Bayliss, P. E.; Carr, C. E.; Johnson, J. L.; Guha, S.; Kobler, P.; Catz, S. D.; Gill, M. et al. Aberrant autolysosomal regulation is linked to the induction of embryonic senescence: Differential roles of Beclin 1 and p53 in vertebrate Spns1 deficiency. PLoS Genet. 2014, 10, e1004409.
Zhu, H. Y.; Li, Q. Q.; Liao, T. P.; Yin, X.; Chen, Q.; Wang, Z. Y.; Dai, M. F.; Yi, L.; Ge, S. Y.; Miao, C. J. et al. Metabolomic profiling of single enlarged lysosomes. Nat. Methods 2021, 18, 788–798.
Fortunato, F.; Bürgers, H.; Bergmann, F.; Rieger, P.; Büchler, M. W.; Kroemer, G.; Werner, J. Impaired autolysosome formation correlates with Lamp-2 depletion: Role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 2009, 137, 350–360.e5.
Eskelinen, E. L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Aspects Med. 2006, 27, 495–502.
Notomi, S.; Ishihara, K.; Efstathiou, N. E.; Lee, J. J.; Hisatomi, T.; Tachibana, T.; Konstantinou, E. K.; Ueta, T.; Murakami, Y.; Maidana, D. E. et al. Genetic LAMP2 deficiency accelerates the age-associated formation of basal laminar deposits in the retina. Proc. Natl. Acad. Sci. USA 2019, 116, 23724–23734.
Langemeyer, L.; Fröhlich, F.; Ungermann, C. Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 2018, 28, 957–970.
Gutierrez, M. G.; Munafó, D. B.; Berón, W.; Colombo, M. I. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004, 117, 2687–2697.
Szatmári, Z.; Sass, M. The autophagic roles of Rab small GTPases and their upstream regulators: A review. Autophagy 2014, 10, 1154–1166.
Park, Y. E.; Hayashi, Y. K.; Bonne, G.; Arimura, T.; Noguchi, S.; Nonaka, I.; Nishino, I. Autophagic degradation of nuclear components in mammalian cells. Autophagy 2009, 5, 795–804.
Lan, Y. Y.; Londoño, D.; Bouley, R.; Rooney, M. S.; Hacohen, N. Dnase2a deficiency uncovers lysosomal clearance of damaged nuclear DNA via autophagy. Cell Rep. 2014, 9, 180–192.
Qiang, L.; Zhao, B. Z.; Shah, P.; Sample, A.; Yang, S.; He, Y. Y. Autophagy positively regulates DNA damage recognition by nucleotide excision repair. Autophagy 2016, 12, 357–368.
Jin, X.; Wang, K. H.; Wang, L.; Liu, W. W.; Zhang, C.; Qiu, Y. X.; Liu, W.; Zhang, H. Y.; Zhang, D.; Yang, Z. X. et al. RAB7 activity is required for the regulation of mitophagy in oocyte meiosis and oocyte quality control during ovarian aging. Autophagy 2022, 18, 643–660.
Snider, M. D. A role for Rab7 GTPase in growth factor-regulated cell nutrition and apoptosis. Mol. Cell 2003, 12, 796–797.
Lv, Y. J.; Yang, Y.; Sui, B. D.; Hu, C. H.; Zhao, P.; Liao, L.; Chen, J.; Zhang, L. Q.; Yang, T. T.; Zhang, S. F. et al. Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice. Theranostics 2018, 8, 2387–2406.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 82170924), the National Key R&D Program of China (No. 2021YFA1100600), the Pearl River Talent Recruitment Program (Nos. 2019ZT08Y485 and 2019JC01Y138), the Guangdong Financial Fund for High-Caliber Hospital Construction (174-2018-XMZC-0001-03-0125, C-03 and D-11), the Sun Yat-sen University Young Teacher Key Cultivation Project (No. 18ykzd05).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4709_MOESM1_ESM.pdf
Apoptotic vesicles rejuvenate mesenchymal stem cells via Rab7-mediated autolysosome formation and alleviate bone loss in aging mice
Rights and permissions
About this article
Cite this article
Lei, F., Huang, Z., Ou, Q. et al. Apoptotic vesicles rejuvenate mesenchymal stem cells via Rab7-mediated autolysosome formation and alleviate bone loss in aging mice. Nano Res. 16, 822–833 (2023). https://doi.org/10.1007/s12274-022-4709-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4709-4