Abstract
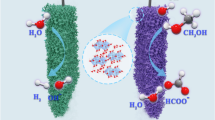
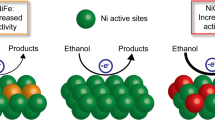
High-entropy multi-elemental (HEM) electrocatalysts present superior catalytic performance due to the efficient synergism of their components. HEM electrocatalysts are usually prepared through hydrothermal reactions or calcination, which could generate undesired heterogeneous structures that hinder the exploration of the structure-property relationship of these HEM electrocatalysts. Herein, we report a sol-gel method to synthesize homogeneous HEM electrocatalysts for electro-oxidation of methanol and urea (methanol oxidation reaction (MOR) and urea oxidation reaction (UOR)), through an acid-catalyzed gelation at room temperature. With Ni as the primary component for MOR and UOR, Co can reduce the overpotentials, while Fe can increase the catalytic activities and durability. Borate and phosphate can tune the charge distribution in active sites and speed up the reaction kinetics through fast proton transfer. Thus, the optimal Ni2Fe0.5Co0.5-BP HEM catalyst demonstrates superior catalytic activity together with good durability and great resistance to CO poisoning. In addition, a direct methanol fuel cell with Ni2Fe0.5Co0.5-BP electrode can not only provide power, but also produce formic acid with high yield and high Faraday efficiency. This work presents a simple strategy to prepare high-performance HEM electrocatalysts for fuel cells and production of value-added chemicals.

Similar content being viewed by others
References
Li, J. R.; Jilani, S. Z.; Lin, H. H.; Liu, X. M.; Wei, K. C.; Jia, Y. K.; Zhang, P.; Chi, M. F.; Tong, Y. Y. J.; Xi, Z. et al. Ternary CoPtAu nanoparticles as a general catalyst for highly efficient electro-oxidation of liquid fuels. Angew. Chem., Int. Ed. 2019, 58, 11527–11533.
Yang, C. Z.; Jiang, Q. G.; Li, W. H.; He, H. Y.; Yang, L.; Lu, Z. Y.; Huang, H. J. Ultrafine Pt nanoparticle-decorated 3D hybrid architectures built from reduced graphene oxide and MXene nanosheets for methanol oxidation. Chem. Mater. 2019, 31, 9277–9287.
Feng, Y.; Liu, H.; Yang, J. A selective electrocatalyst-based direct methanol fuel cell operated at high concentrations of methanol. Sci. Adv. 2017, 3, e1700580.
Yang, L.; Li, G. Q.; Ma, R. P.; Hou, S.; Chang, J. F.; Ruan, M. B.; Cai, W. B.; Jin, Z.; Xu, W. L.; Wang, G. L. et al. Nanocluster PtNiP supported on graphene as an efficient electrocatalyst for methanol oxidation reaction. Nano Res. 2021, 14, 2853–2860.
Yang, C. Z.; Jiang, Q. G.; Liu, H.; Yang, L.; He, H. Y.; Huang, H. J.; Li, W. H. Pt-on-Pd bimetallic nanodendrites stereoassembled on MXene nanosheets for use as high-efficiency electrocatalysts toward the methanol oxidation reaction. J. Mater. Chem. A 2021, 9, 15432–15440.
Chen, F.; Gao, L. L.; Zhai, M. X.; Wu, N.; Zhang, X.; Guo, R. H.; Ma, M. M.; Hu, T. P. Carbon monoxide-resistant copper-cobalt nanocrystal@nitrogen-doped carbon electrocatalysts for methanol oxidation reaction. J. Alloys Compd. 2021, 888, 161563.
Liu, C.; Zhou, W.; Zhang, J. F.; Chen, Z. L.; Liu, S. L.; Zhang, Y.; Yang, J. X.; Xu, L. Y.; Hu, W. B.; Chen, Y. N. et al. Air-assisted transient synthesis of metastable nickel oxide boosting alkaline fuel oxidation reaction. Adv. Energy Mater. 2020, 10, 2001397.
Zhai, M. X.; Chen, F.; Wu, N.; Zhang, X.; Guo, R. H.; Ma, M. M.; Hu, T. P. Highly conductive and CO-resistant cobalt-based monolithic electrodes for the catalytic oxidation of methanol. ChemElectroChem 2021, 8, 4854–4860.
Wang, X. P.; Xi, S. B.; Lee, W. S. V.; Huang, P. R.; Cui, P.; Zhao, L.; Hao, W. C.; Zhao, X. S.; Wang, Z. B.; Wu, H. J. et al. Materializing efficient methanol oxidation via electron delocalization in nickel hydroxide nanoribbon. Nat. Commun. 2020, 11, 4647.
Huang, H. J.; Wei, Y. J.; Yang, Y.; Yan, M. M.; He, H. Y.; Jiang, Q. G.; Yang, X. F.; Zhu, J. X. Controllable synthesis of grain boundary-enriched Pt nanoworms decorated on graphitic carbon nanosheets for ultrahigh methanol oxidation catalytic activity. J. Energy Chem. 2021, 57, 601–609.
Zhu, X. J.; Dou, X. Y.; Dai, J.; An, X. D.; Guo, Y. Q.; Zhang, L. D.; Tao, S.; Zhao, J. Y.; Chu, W. S.; Zeng, X. C. et al. Metallic nickel hydroxide nanosheets give superior electrocatalytic oxidation of urea for fuel cells. Angew. Chem., Int. Ed. 2016, 55, 12465–12469.
Liu, Z.; Zhang, C. Z.; Liu, H.; Feng, L. G. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl. Catal. B: Environ. 2020, 276, 119165.
Dubale, A. A.; Zheng, Y. Y.; Wang, H. L.; Hübner, R.; Li, Y.; Yang, J.; Zhang, J. W.; Sethi, N. K.; He, L. Q.; Zheng, Z. K. et al. High-performance bismuth-doped nickel aerogel electrocatalyst for the methanol oxidation reaction. Angew. Chem., Int. Ed. 2020, 59, 13891–13899.
Li, Q.; Li, X. R.; Gu, J. W.; Li, Y. L.; Tian, Z. Q.; Pang, H. Porous rod-like Ni2P/Ni assemblies for enhanced urea electrooxidation. Nano Res. 2021, 14, 1405–1412.
Wang, L.; Liu, Z. P.; Zhu, S. Q.; Shao, M. H.; Yang, B. L.; Chen, J. G. Tungsten carbide and cobalt modified nickel nanoparticles supported on multiwall carbon nanotubes as highly efficient electrocatalysts for urea oxidation in alkaline electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 41338–41343.
Li, J. S.; Wei, R. L.; Wang, X.; Zuo, Y.; Han, X.; Arbiol, J.; Llorca, J.; Yang, Y. Y.; Cabot, A.; Cui, C. H. Selective methanol-to-formate electrocatalytic conversion on branched nickel carbide. Aggew. Chem., Int. Ed. 2020, 55, 20826–20830.
Du, J. N.; You, S. J.; Li, X. R.; Tang, B.; Jiang, B. J.; Yu, Y.; Cai, Z.; Ren, N. Q.; Zou, J. L. In situ crystallization of active NiOOH/CoOOH heterostructures with hydroxide ion adsorption sites on velutipes-like CoSe/NiSe nanorods as catalysts for oxygen evolution and cocatalysts for methanol oxidation. ACS Appl. Mater. Interfaces 2020, 12, 686–697.
Wu, Y. P.; Tian, J. W.; Liu, S.; Li, B.; Zhao, J.; Ma, L. F.; Li, D. S.; Lan, Y. Q.; Bu, X. H. Bi-microporous metal-organic frameworks with cubane [M4(OH)4] (M = Ni, Co) clusters and pore-space partition for electrocatalytic methanol oxidation reaction. Angew. Chem. 2019, 131, 12313–12317.
Xu, W.; Chen, H.; Jie, K. C.; Yang, Z. Z.; Li, T. T.; Dai, S. Entropy-driven mechanochemical synthesis of polymetallic zeolitic imidazolate frameworks for CO2 fixation. Angew. Chem., Int. Ed. 2019, 58, 5018–5022.
Liu, M. M.; Zhang, Z.; Okejiri, F.; Yang, S. Z.; Zhou, S. H.; Dai, S. Entropy-maximized synthesis of multimetallic nanoparticle catalysts via a ultrasonication-assisted wet chemistry method under ambient conditions. Adv. Mater. Interfaces 2019, 6, 1900015.
Nutor, R. K.; Cao, Q. P.; Wang, X. D.; Zhang, D. X.; Fang, Y. Z.; Zhang, Y.; Jiang, J. Z. Phase selection, lattice distortions, and mechanical properties in high-entropy alloys. Adv. Eng. Mater. 2020, 22, 2000466.
Xin, Y.; Li, S. H.; Qian, Y. Y.; Zhu, W. K.; Yuan, H. B.; Jiang, P. Y.; Guo, R. H.; Wang, L. B. High-entropy alloys as a platform for catalysis: Progress, challenges, and opportunities. ACS Catal. 2020, 10, 11280–11306.
Chang, X. J.; Zeng, M. Q.; Liu, K. L.; Fu, L. Phase engineering of high-entropy alloys. Adv. Mater. 2020, 32, 1907226.
Bondesgaard, M.; Broge, N. L. N.; Mamakhel, A.; Bremholm, M.; Iversen, B. B. General solvothermal synthesis method for complete solubility range bimetallic and high-entropy alloy nanocatalysts. Adv. Funct. Mater. 2019, 29, 1905933.
Yao, Y. G.; Huang, Z. N.; Xie, P. F.; Lacey, S. D.; Jacob, R. J.; Xie, H.; Chen, F. J.; Nie, A. M.; Pu, T. C.; Rehwoldt, M. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 355, 1489–1494.
Warren, S. C.; Perkins, M. R.; Adams, A. M.; Kamperman, M.; Burns, A. A.; Arora, H.; Herz, E.; Suteewong, T.; Sai, H.; Li, Z. H. et al. A silica sol-gel design strategy for nanostructured metallic materials. Nat. Mater. 2012, 11, 460–467.
Zhang, B.; Wang, L.; Cao, Z.; Kozlov, S. M.; García de Arquer, F. P.; Dinh, C. T.; Li, J.; Wang, Z. Y.; Zheng, X. L.; Zhang, L. S. et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 2020, 3, 985–992.
Fan, J.; Boettcher, S. W.; Stucky, G. D. Nanoparticle assembly of ordered multicomponent mesostructured metal oxides via a versatile sol-gel process. Chem. Mater. 2006, 18, 6391–6396.
Zhang, B.; Zheng, X. L.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L. L.; Xu, J. X.; Liu, M.; Zheng, L. R. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337.
Abdullah, M. I.; Hameed, A.; Zhang, N.; Ma, M. M. Ultrasonic-assisted synthesis of amorphous polyelemental hollow nanoparticles as efficient and stable bifunctional electrocatalysts for overall water splitting. Adv. Mater. Interfaces 2019, 6, 1900586.
Abdullah, M. I.; Hameed, A.; Hu, T.; Zhang, N.; Ma, M. Crystalline multi-metal nanosheets array with enriched oxygen vacancies as efficient and stable bifunctional electrocatalysts for water splitting. ACS Appl. Energy Mater. 2019, 2, 8919–8929.
Kim, J. W.; Augustyn, V.; Dunn, B. The effect of crystallinity on the rapid pseudocapacitive response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148.
Danks, A. E.; Hall, S. R.; Schnepp, Z. The evolution of “sol-gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112.
Chen, J. Y. C.; Miller, J. T.; Gerken, J. B.; Stahl, S. S. Inverse spinel NiFeAlO4 as a highly active oxygen evolution electrocatalyst: Promotion of activity by a redox-inert metal ion. Energy Environ. Sci. 2014, 7, 1382–1386.
Liang, L.; Yu, F. K.; An, Y. R.; Liu, M. M.; Zhou, M. H. Preparation of transition metal composite graphite felt cathode for efficient heterogeneous electro-Fenton process. Environ. Sci. Pollut. Res. 2017, 24, 1122–1132.
Kan, W. H.; Chen, M.; Bae, J. S.; Kim, B. H.; Thangadurai, V. Determination of Fe oxidation states in the B-site ordered perovskite-type Ba2Ca0.67Fe0.33NbO6-δ at the surface (nano-scale) and bulk by variable temperature XPS and TGA and their impact on electrochemical catalysis. J. Mater. Chem. A 2014, 2, 8736–8741.
Feng, X. G.; Wang, H. X.; Bo, X. J.; Guo, L. P. Bimetal-organic framework-derived porous rodlike cobalt/nickel nitride for all-pH value electrochemical hydrogen evolution. ACS Appl. Mater. Interfaces 2019, 11, 8018–8024.
Tan, S. J. R.; Abdelwahab, I.; Chu, L. Q.; Poh, S. M.; Liu, Y. P.; Lu, J.; Chen, W.; Loh, K. P. Quasi-monolayer black phosphorus with high mobility and air stability. Adv. Mater. 2018, 30, 1704619.
Cao, E. P.; Chen, Z. M.; Wu, H.; Yu, P.; Wang, Y.; Xiao, F.; Chen, S.; Du, S. C.; Xie, Y.; Wu, Y. Q. et al. Boron-induced electronic-structure reformation of CoP nanoparticles drives enhanced pH-universal hydrogen evolution. Angew. Chem., Int. Ed. 2020, 59, 4154–4160.
Wang, S. L.; Yang, X. D.; Liu, Z.; Yang, D. W.; Feng, L. G. Efficient nanointerface hybridization in a nickel/cobalt oxide nanorod bundle structure for urea electrolysis. Nanoscale 2020, 12, 10827–10833.
Abdullah, M. I.; Hameed, A.; Zhang, N.; Islam, M. H.; Ma, M. M.; Pollet, B. G.; Ma, M. Ultrasonically surface-activated nickel foam as a highly efficient monolith electrode for the catalytic oxidation of methanol to formate. ACS Appl. Mater. Interfaces 2021, 13, 30603–30613.
Cui, X.; Xiao, P.; Wang, J.; Zhou, M.; Guo, W. L.; Yang, Y.; He, Y. J.; Wang, Z. W.; Yang, Y. K.; Zhang, Y. H. et al. Highly branched metal alloy networks with superior activities for the methanol oxidation reaction. Angew. Chem. 2017, 125, 4559–4564.
Rezaee, S.; Shahrokhian, S. Facile synthesis of petal-like NiCo/NiO-CoO/nanoporous carbon composite based on mixed-metallic MOFs and their application for electrocatalytic oxidation of methanol. Appl. Catal. B: Environ. 2019, 244, 802–813.
Ullah, N.; Xie, M.; Oluigbo, C. J.; Xu, Y. G.; Xie, J. M.; Rasheed, H. U.; Zhang, M. M. Nickel and cobalt in situ grown in 3-dimensional hierarchical porous graphene for effective methanol electro-oxidation reaction. J. Electroanal. Chem. 2019, 838, 7–15.
Baksi, A.; Nandam, S. H.; Wang, D.; Kruk, R.; Chellali, M. R.; Ivanisenko, J.; Gallino, I.; Hahn, H.; Bag, S. Ni60Nb40 nanoglass for tunable magnetism and methanol oxidation. ACS Appl. Nano Mater. 2020, 3, 7252–7259.
Xiong, L. K.; Sun, Z. T.; Zhang, X.; Zhao, L.; Huang, P.; Chen, X. W.; Jin, H. D.; Sun, H.; Lian, Y. B.; Deng, Z. et al. Octahedral gold-silver nanoframes with rich crystalline defects for efficient methanol oxidation manifesting a CO-promoting effect. Nat. Commun. 2019, 10, 3782.
Pieta, I. S.; Rathi, A.; Pieta, P.; Nowakowski, R.; Holdynski, M.; Pisarek, M.; Kaminska, A.; Gawande, M. B.; Zboril, R. Electrocatalytic methanol oxidation over Cu, Ni and Bimetallic Cu-Ni nanoparticles supported on graphitic carbon nitride. Appl. Catal. B: Environ. 2019, 244, 272–283.
Hao, J.; Liu, J. W.; Wu, D.; Chen, M. X.; Liang, Y.; Wang, Q.; Wang, L.; Fu, X. Z.; Luo, J. L. In situ facile fabrication of Ni(OH)2 nanosheet arrays for electrocatalytic Co-production of formate and hydrogen from methanol in alkaline solution. Appl. Catal. B: Environ. 2021, 281, 119510.
Anu Prathap, M. U.; Srivastava, R. Synthesis of NiCo2O4 and its application in the electrocatalytic oxidation of methanol. Nano Energy 2013, 2, 1046–1053.
Anantharaj, S.; Sugime, H.; Noda, S. Ultrafast growth of a Cu(OH)2-CuO nanoneedle array on Cu foil for methanol oxidation electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 27327–27338.
Cui, X.; Guo, W.; Zhou, M.; Yang, Y.; Li, Y.; Xiao, P.; Zhang, Y.; Zhang, X. Promoting effect of Co in NimCon (m + n = 4) bimetallic electrocatalysts for methanol oxidation reaction. ACS Appl. Mater. Interfaces 2015, 7, 493–503.
Guo, F.; Ye, K.; Cheng, K.; Wang, G. L.; Cao, D. X. Preparation of nickel nanowire arrays electrode for urea electro-oxidation in alkaline medium. J. Power Sources 2015, 278, 562–568.
Chen, F.; Wu, N.; Zhai, M. X.; Zhang, X.; Guo, R. H.; Hu, T. P.; Ma, M. M. Robust copper nanocrystal/nitrogen-doped carbon monoliths as carbon monoxide-resistant electrodes for methanol oxidation reaction. J. Energy Chem. 2021, 58, 247–255.
Forslund, R. P.; Alexander, C. T.; Abakumov, A. M.; Johnston, K. P.; Stevenson, K. J. Enhanced electrocatalytic activities by substitutional tuning of nickel-based ruddlesden-popper catalysts for the oxidation of urea and small alcohols. ACS Catal. 2019, 9, 2664–2673.
Li, D.; Huang, L. L.; Tian, Y.; Liu, T. T.; Zhen, L.; Feng, Y. J. Facile synthesis of porous Cu-Sn alloy electrode with prior selectivity of formate in a wide potential range for CO2 electrochemical reduction. Appl. Catal. B: Environ. 2021, 292, 120119.
Wu, N.; Zhai, M. X.; Chen, F.; Zhang, X.; Guo, R. H.; Hu, T. P.; Ma, M. M. Nickel nanocrystal/nitrogen-doped carbon composites as efficient and carbon monoxide-resistant electrocatalysts for methanol oxidation reactions. Nanoscale 2020, 12, 21687–21694.
Zhai, M. X.; Chen, F.; Wu, N.; Guo, R. H.; Zhang, X.; Hu, T. P.; Ma, M. M. Porous layered cobalt nanocrystal/nitrogen-doped carbon composites as efficient and CO-resistant electrocatalysts for methanol oxidation reaction. Appl. Surf. Sci. 2021, 545, 149016.
Cao, C. S.; Ma, D. D.; Jia, J. C.; Xu, Q.; Wu, X. T.; Zhu, Q. L. Divergent paths, same goal: A pair-electrosynthesis tactic for cost-efficient and exclusive formate production by metal-organic-framework-derived 2D electrocatalysts. Adv. Mater. 2021, 33, 2008631.
Xiang, K.; Wu, D.; Deng, X. H.; Li, M.; Chen, S. Y.; Hao, P. P.; Guo, X. F.; Luo, J. L.; Fu, X. Z. Boosting H2 generation coupled with selective oxidation of methanol into value-added chemical over cobalt hydroxide@hydroxysulfide nanosheets electrocatalysts. Adv. Funct. Mater. 2020, 30, 1909610.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21778052 and 21975240) and by the Fundamental Research Funds for the Central Universities (No. WK2060190102).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Mushiana, T., Khan, M., Abdullah, M.I. et al. Facile sol-gel preparation of high-entropy multielemental electrocatalysts for efficient oxidation of methanol and urea. Nano Res. 15, 5014–5023 (2022). https://doi.org/10.1007/s12274-022-4186-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4186-9