Abstract
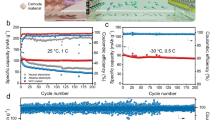
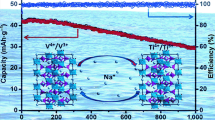
Aqueous rechargeable sodium ion batteries (ARSIBs), with intrinsic safety, low cost, and greenness, are attracting more and more attentions for large scale energy storage application. However, the low energy density hampers their practical application. Here, a battery architecture designed by bipolar electrode with graphite/amorphous carbon film as current collector shows high energy density and excellent rate-capability. The bipolar electrode architecture is designed to not only improve energy density of practical battery by minimizing inactive ingredient, such as tabs and cases, but also guarantee high rate-capability through a short electron transport distance in the through-plane direction instead of in-plane direction for traditional cell architecture. As a proof of concept, a prototype pouch cell of 8 V based on six Na2MnFe(CN)6∥NaTi2(PO4)3 bipolar electrodes stacking using a “water-in-polymer” gel electrolyte is demonstrated to cycle up to 4,000 times, with a high energy density of 86 Wh·kg−1 based on total mass of both cathode and anode. This result opens a new avenue to develop advance high-energy ARSIBs for grid-scale energy storage applications.

Similar content being viewed by others
References
Liu, Z. X.; Huang, Y.; Huang, Y.; Yang, Q.; Li, X. L.; Huang, Z. D.; Zhi, C. Y. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 2020, 49, 180–232.
Yang, C. Y.; Chen, J.; Ji, X.; Pollard, T. P.; Lü, X. J.; Sun, C. J.; Hou, S.; Liu, Q.; Liu, C. M.; Qing, T. T. et al. Aqueous Li-ion battery enabled by halogen conversion-intercalation chemistry in graphite. Nature 2019, 569, 245–250.
Suo, L. M.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X. L.; Luo, C.; Wang, C. S.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943.
Gupta, S. From gadgets to the smart grid. Nature 2015, 526, S90–S91.
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R. K.; Yadav, R. M.; Verma, R. K.; Singh, D. P.; Tan, W. K.; Pérez del Pino, A.; Moshkalev, S. A. et al. A review on synthesis of graphene, h-BN and MoS2 for energy storage applications: Recent progress and perspectives. Nano Res. 2019, 12, 2655–2694.
Larcher, D.; Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29.
Hueso, K. B.; Palomares, V.; Armand, M.; Rojo, T. Challenges and perspectives on high and intermediate-temperature sodium batteries. Nano Res. 2017, 10, 4082–4114.
Hasa, I.; Hassoun, J.; Passerini, S. Nanostructured Na-ion and Li-ion anodes for battery application: A comparative overview. Nano Res. 2017, 10, 3942–3969.
Janek, J.; Zeier, W. G. A solid future for battery development. Nat. Energy 2016, 1, 16141.
Xu, K.; Wang, C. Batteries: Widening voltage windows. Nat. Energy 2016, 1, 16161.
Liu, J. L.; Xu, C. H.; Chen, Z.; Ni, S. B.; Shen, Z. X. Progress in aqueous rechargeable batteries. Green Energy Environ. 2018, 3, 20–41.
Eftekhari, A. High-energy aqueous lithium batteries. Adv. Energy Mater. 2018, 8, 1801156.
Bin, D.; Wang, F.; Tamirat, A. G.; Suo, L. M.; Wang, Y. G.; Wang, C. S.; Xia, Y. Y. Progress in aqueous rechargeable sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1703008.
Liu, M.; Ao, H.; Jin, Y.; Hou, Z.; Zhang, X.; Zhu, Y.; Qian, Y. Aqueous rechargeable sodium ion batteries: Developments and prospects. Mater. Today Energy 2020, 17, 100432.
Kim, H.; Hong, J.; Park, K. Y.; Kim, H.; Kim, S. W.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827.
Chao, D. L.; Zhou, W. H.; Xie, F. X.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S. Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098.
Chao, D. L.; Qiao, S. Z. Toward high-voltage aqueous batteries: Super-or low-concentrated electrolyte? Joule 2020, 4, 1846–1851.
Li, Z.; Young, D.; Xiang, K.; Carter, W. C.; Chiang, Y. M. Towards high power high energy aqueous sodium-ion batteries: The NaTi2(PO4)3/Na0.44MnO2 system. Adv. Energy Mater. 2013, 3, 290–294.
Whitacre, J. F.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commun. 2010, 12, 463–466.
Whitacre, J. F.; Wiley, T.; Shanbhag, S.; Wenzhuo, Y.; Mohamed, A.; Chun, S. E.; Weber, E.; Blackwood, D.; Lynch-Bell, E.; Gulakowski, J. et al. An aqueous electrolyte, sodium ion functional, large format energy storage device for stationary applications. J. Power Sources 2012, 213, 255–264.
Whitacre, J. F.; Shanbhag, S.; Mohamed, A.; Polonsky, A.; Carlisle, K.; Gulakowski, J.; Wu, W.; Smith, C.; Cooney, L.; Blackwood, D. et al. A polyionic, large-format energy storage device using an aqueous electrolyte and thick-format composite NaTi2(PO4)3/activated carbon negative electrodes. Energy Technol. 2015, 3, 20–31.
Wu, X. Y.; Cao, Y. L.; Ai, X. P.; Qian, J. F.; Yang, H. X. A low-cost and environmentally benign aqueous rechargeable sodium-ion battery based on NaTi2(PO4)3-Na2NiFe(CN)6 intercalation chemistry. Electrochem. Commun. 2013, 31, 145–148.
Wu, X. Y.; Sun, M. Y.; Shen, Y. F.; Qian, J. F.; Cao, Y. L.; Ai, X. P.; Yang, H. X. Energetic aqueous rechargeable sodium-ion battery based on Na2CuFe(CN)6-NaTi2(PO4)3 intercalation chemistry. ChemSusChem 2014, 7, 407–411.
Pasta, M.; Wessells, C. D.; Liu, N.; Nelson, J.; McDowell, M. T.; Huggins, R. A.; Toney, M. F.; Cui, Y. Full open-framework batteries for stationary energy storage. Nat. Commun. 2014, 5, 3007.
Kumar, P. R.; Jung, Y. H.; Lim, C. H.; Kim, D. K. Na3V2O2x(PO4)2F3−2x: A stable and high-voltage cathode material for aqueous sodium-ion batteries with high energy density. J. Mater. Chem. A 2015, 3, 6271–6275.
Zhang, Q.; Liao, C. Y.; Zhai, T. Y.; Li, H. Q. A high rate 1. 2V aqueous sodium-ion battery based on all NASICON structured NaTi2(PO4)3 and Na3V2(PO4)3. Electrochim. Acta 2016, 196, 470–478.
Gao, H. C.; Goodenough, J. B. An aqueous symmetric sodium-ion battery with NASICON-structured Na3MnTi(PO4)3. Angew. Chem., Int. Ed. 2016, 55, 12768–12772.
Wang, Y. S.; Mu, L. Q.; Liu, J.; Yang, Z. Z.; Yu, X. Q.; Gu, L.; Hu, Y. S.; Li, H.; Yang, X. Q.; Chen, L. Q. et al. A novel high capacity positive electrode material with tunnel-type structure for aqueous sodium-ion batteries. Adv. Energy Mater. 2015, 5, 1501005.
Suo, L. M.; Borodin, O.; Wang, Y. S.; Rong, X. H.; Sun, W.; Fan, X.; Xu, S. Y.; Schroeder, M. A.; Cresce, A. V.; Wang, F. et al. “Water-in-salt” electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting. Adv. Energy Mater. 2017, 7, 1701189.
Zhang, Q. C.; Man, P.; He, B.; Li, C. W.; Li, Q. L.; Pan, Z. H.; Wang, Z. X.; Yang, J.; Wang, Z.; Zhou, Z. Y. et al. Binder-free NaTi2(PO4)3 anodes for high-performance coaxial-fiber aqueous rechargeable sodium-ion batteries. Nano Energy 2020, 67, 104212.
Chen, L.; Shao, H. Z.; Zhou, X. F.; Liu, G. Q.; Jiang, J.; Liu, Z. P. Water-mediated cation intercalation of open-framework indium hexacyanoferrate with high voltage and fast kinetics. Nat. Commun. 2016, 7, 11982.
Sun, T. J.; Liu, C.; Wang, J. Y.; Nian, Q. S.; Feng, Y. Z.; Zhang, Y.; Tao, Z. L.; Chen, J. A phenazine anode for high-performance aqueous rechargeable batteries in a wide temperature range. Nano Res. 2020, 13, 676–683.
Wang, H. B.; Zhang, T. R.; Chen, C.; Ling, M.; Lin, Z.; Zhang, S. Q.; Pan, F.; Liang, C. D. High-performance aqueous symmetric sodium-ion battery using NASICON-structured Na2VTi(PO4)3. Nano Res. 2018, 11, 490–498.
Perticarari, S.; Grange, E.; Doizy, T.; Pellegrin, Y.; Quarez, E.; Oyaizu, K.; Fernandez-Ropero, A. J.; Guyomard, D.; Poizot, P.; Odobel, F. et al. Full organic aqueous battery based on TEMPO small molecule with millimeter-thick electrodes. Chem. Mater. 2019, 31, 1869–1880.
Liu, S.; Wang, L. B.; Liu, J.; Zhou, M.; Nian, Q. S.; Feng, Y. Z.; Tao, Z. L.; Shao, L. Y. Na3V2(PO4)2F3-SWCNT: A high voltage cathode for non-aqueous and aqueous sodium-ion batteries. J. Mater. Chem. A 2019, 7, 248–256.
Jiang, L. W.; Liu, L. L.; Yue, J. M.; Zhang, Q. Q.; Zhou, A. X.; Borodin, O.; Suo, L. M.; Li, H.; Chen, L. Q.; Xu, K. et al. High-voltage aqueous na-ion battery enabled by inert-cation-assisted water-in-salt electrolyte. Adv. Mater. 2020, 32, 1904427.
Han, J.; Zarrabeitia, M.; Mariani, A.; Jusys, Z.; Hekmatfar, M.; Zhang, H.; Geiger, D.; Kaiser, U.; Behm, R. J.; Varzi, A. et al. Halide-free water-in-salt electrolytes for stable aqueous sodium-ion batteries. Nano Energy 2020, 77, 105176.
Luo, D. X.; Lei, P.; Tian, G. R.; Huang, Y. X.; Ren, X. F.; Xiang, X. D. Insight into electrochemical properties and reaction mechanism of a cobalt-rich prussian blue analogue cathode in a NaSO3CF3 electrolyte for aqueous sodium-ion batteries. J. Phys. Chem. C 2020, 124, 5958–5965.
Nakamoto, K.; Sakamoto, R.; Sawada, Y.; Ito, M.; Okada, S. Over 2 V aqueous sodium-ion battery with prussian blue-type electrodes. Small Methods 2019, 3, 1800220.
Yang, C. Y.; Chen, J.; Qing, T. T.; Fan, X. L.; Sun, W.; Von Cresce, A.; Ding, M. S.; Borodin, O.; Vatamanu, J.; Schroeder, M. A. et al. 4.0 V aqueous Li-ion batteries. Joule 2017, 1, 122–132.
Warner, J. The Handbook of Lithium-Ion Battery Pack Design: Chemistry, Components, Types and Terminology; Elsevier: Amsterdam, 2015.
Pistoia, G. Lithium-Ion Batteries; Elsevier: Amsterdam, 2013.
Ellis, K.; Hill, A.; Hill, J.; Loyns, A.; Partington, T. The performance of Ebonex® electrodes in bipolar lead-acid batteries. J. Power Sources 2004, 136, 366–371.
Wiesener, K.; Ohms, D.; Benczúr-Ürmössy, G.; Berthold, M.; Haschka, F. High power metal hydride bipolar battery. J. Power Sources 1999, 84, 248–258.
Ogihara, N.; Yasuda, T.; Kishida, Y.; Ohsuna, T.; Miyamoto, K.; Ohba, N. Organic dicarboxylate negative electrode materials with remarkably small strain for high-voltage bipolar batteries. Angew. Chem. 2014, 126, 11651–11656.
Liu, T. F.; Zhang, Y. P.; Chen, C.; Lin, Z.; Zhang, S. Q.; Lu, J. Sustainability-inspired cell design for a fully recyclable sodium ion battery. Nat. Commun. 2019, 10, 1965.
Luo, W.; Hayden, J.; Jang, S. H.; Wang, Y. L.; Zhang, Y.; Kuang, Y. D.; Wang, Y. B.; Zhou, Y. B.; Rubloff, G. W.; Lin, C. F. et al. Highly conductive, light weight, robust, corrosion-resistant, scalable, all-fiber based current collectors for aqueous acidic batteries. Adv. Energy Mater. 2018, 8, 1702615.
Yao, Y. G.; Jiang, F.; Yang, C. Y.; Fu, K. K.; Hayden, J.; Lin, C. F.; Xie, H.; Jiao, M. L.; Yang, C. P.; Wang, Y. L. et al. Epitaxial welding of carbon nanotube networks for aqueous battery current collectors. ACS Nano 2018, 12, 5266–5273.
Yun, Q. B.; He, Y. B.; Lv, W.; Zhao, Y.; Li, B. H.; Kang, F. Y.; Yang, Q. H. Chemical dealloying derived 3D porous current collector for Li metal anodes. Adv. Mater. 2016, 28, 6932–6939.
Whitehead, A. H.; Schreiber, M. Current collectors for positive electrodes of lithium-based batteries. J. Electrochem. Soc. 2005, 152, A2105.
Gheytani, S.; Liang, Y. L.; Jing, Y.; Xu, J. Q.; Yao, Y. Chromate conversion coated aluminium as a light-weight and corrosion-resistant current collector for aqueous lithium-ion batteries. J. Mater. Chem. A 2016, 4, 395–399.
Kühnel, R. S.; Reber, D.; Remhof, A.; Figi, R.; Bleiner, D.; Battaglia, C. “Water-in-salt” electrolytes enable the use of cost-effective aluminum current collectors for aqueous high-voltage batteries. Chem. Commun. 2016, 52, 10435–10438.
Wen, Y. H.; Shao, L.; Zhao, P. C.; Wang, B. Y.; Cao, G. O.; Yang, Y. S. Carbon coated stainless steel mesh as a low-cost and corrosion-resistant current collector for aqueous rechargeable batteries. J. Mater. Chem. A 2017, 5, 15752–15758.
Wang, Y. G.; Xia, Y. Y. A new concept hybrid electrochemical surpercapacitor: Carbon/LiMn2O4 aqueous system. Electrochem. Commun. 2005, 7, 1138–1142.
Luo, J. Y.; Cui, W. J.; He, P.; Xia, Y. Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765.
Liu, S.; Ye, S. H.; Li, C. Z.; Pan, G. L.; Gao, X. P. Rechargeable aqueous lithium-ion battery of TiO2/LiMn2O4 with a high voltage. J. Electrochem. Soc. 2011, 158, A1490.
Ferrari, A. C.; Basko, D. M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52102261), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 20KJB150007), the Natural Science Foundation of Jiangsu Province (No. BK20210942), the Applied Basic Research Programs of Changzhou (No. CJ20200034), and Changzhou Science and Technology Young Talents Promotion Project (No. KYZ21005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hou, Z., Mao, W., Zhang, Z. et al. Bipolar electrode architecture enables high-energy aqueous rechargeable sodium ion battery. Nano Res. 15, 5072–5080 (2022). https://doi.org/10.1007/s12274-022-4113-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4113-0