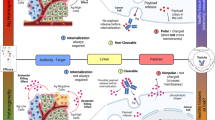
Abstract
Tumor associated macrophages (TAMs) tend to exhibit tumor-promoting M2 phenotype and contribute to the development of immunosuppressive microenvironment of solid tumors. Reprograming TAMs from M2 into tumoricidal M1 phenotype is robust for stimulating tumor immunosuppressive microenvironment (TIME). In this study, we developed a poly(amidoamine) (PAMAM) derivative dendrimer (denoted as fourth generation-N,N-diethylaminoethyl (G4-DEEA)) for efficient loading of Toll-like receptor 7 and 8 (TLR7/8) agonist (R848) to remodel the TIME for potent cancer immunotherapy. G4-DEEA exhibited a high loading capacity of R848 up to 35.9 wt% by taking advantage of its dendritic structure. The resulting formulation (designated as G4-DEEA@R848) effectively polarized M2 macrophages into M1 phenotype in vitro, and improved the maturation and activation of antigen-presenting cells. In the 4T1 orthotopic breast cancer model, G4-DEEA@R848 showed a stronger tumor inhibitory effect than free drug. The mechanistic studies suggested that G4-DEEA@R848 could significantly stimulate the TIME by repolarizing TAMs into M1 phenotype, reducing the presence of immunosuppressive myeloid cells and increasing the infiltration of tumor cytotoxic T cells. This study provides a simple but effective dendrimer-based strategy to improve the formulation of R848 for improved cancer immunotherapy.

Similar content being viewed by others
References
Binnewies, M.; Roberts, E. W.; Kersten, K.; Chan, V.; Fearon, D. F.; Merad, M.; Coussens, L. M.; Gabrilovich, D. I.; Ostrand-Rosenberg, S.; Hedrick, C. C. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550.
Hinshaw, D. C.; Shevde, L. A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566.
Popel, A. S. Immunoactivating the tumor microenvironment enhances immunotherapy as predicted by integrative computational model. Proc. Natl. Acad. Sci. USA 2020, 117, 4447–4449.
Rodell, C. B.; Arlauckas, S. P.; Cuccarese, M. F.; Garris, C. S.; Li, R.; Ahmed, M. S.; Kohler, R. H.; Pittet, M. J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588.
De Palma, M.; Lewis, C. E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013, 23, 277–286.
Guerriero, J. L. Macrophages: The road less traveled, changing anticancer therapy. Trends Mol. Med. 2018, 24, 472–489.
Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; van den Bossche, J.; Mack, M.; Pipeleers, D.; In’t Veld, P.; De Baetselier, P. et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from ly6c(high) monocytes. Cancer Res. 2010, 70, 5728–5739.
Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555.
Biswas, S. K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896.
Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014, 6, 1670–1690.
Bronte, V.; Murray, P. J. Understanding local macrophage phenotypes in disease: Modulating macrophage function to treat cancer. Nat. Med. 2015, 21, 117–119.
DeNardo, D. G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382.
Pyonteck, S. M.; Akkari, L.; Schuhmacher, A. J.; Bowman, R. L.; Sevenich, L.; Quail, D. F.; Olson, O. C.; Quick, M. L.; Huse, J. T.; Teijeiro, V. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272.
Hughes, R.; Qian, B. Z.; Rowan, C.; Muthana, M.; Keklikoglou, I.; Olson, O. C.; Tazzyman, S.; Danson, S.; Addison, C.; Clemons, M. et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015, 75, 3479–3491.
Arwert, E. N.; Harney, A. S.; Entenberg, D.; Wang, Y. R.; Sahai, E.; Pollard, J. W.; Condeelis, J. S. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018, 23, 1239–1248.
Cassetta, L.; Pollard, J. W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904.
Kulkarni, A.; Chandrasekar, V.; Natarajan, S. K.; Ramesh, A.; Pandey, P.; Nirgud, J.; Bhatnagar, H.; Ashok, D.; Ajay, A. K.; Sengupta, S. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat. Biomed. Eng. 2018, 2, 589–599.
Ni, K. Y.; Luo, T. K.; Culbert, A.; Kaufmann, M.; Jiang, X. M.; Lin, W. B. Nanoscale metal-organic framework co-delivers TLR-7 agonists and anti-cd47 antibodies to modulate macrophages and orchestrate cancer immunotherapy. J. Am. Chem. Soc. 2020, 142, 12579–12584.
Guerriero, J. L.; Sotayo, A.; Ponichtera, H. E.; Castrillon, J. A.; Pourzia, A. L.; Schad, S.; Johnson, S. F.; Carrasco, R. D.; Lazo, S.; Bronson, R. T. et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 2017, 543, 428–432.
Panni, R. Z.; Herndon, J. M.; Zuo, C.; Hegde, S.; Hogg, G. D.; Knolhoff, B. L.; Breden, M. A.; Li, X. B.; Krisnawan, V. E.; Khan, S. Q. et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci. Transl. Med. 2019, 11, eaau9240.
Michaelis, K. A.; Norgard, M. A.; Zhu, X. X.; Levasseur, P. R.; Sivagnanam, S.; Liudahl, S. M.; Burfeind, K. G.; Olson, B.; Pelz, K. R.; Angeles Ramos, D. M. et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019, 10, 4682.
Liu, L. Q.; Wang, Y.; Guo, X.; Zhao, J. Y.; Zhou, S. B. A biomimetic polymer magnetic nanocarrier polarizing tumor-associated macrophages for potentiating immunotherapy. Small 2020, 16, 2003543.
Jurk, M.; Heil, F.; Vollmer, J.; Schetter, C.; Krieg, A. M.; Wagner, H.; Lipford, G.; Bauer, S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound r-848. Nat. Immunol. 2002, 3, 499.
Krieg, A. M.; Vollmer, J. Toll-like receptors 7, 8, and 9: Linking innate immunity to autoimmunity. Immunol. Rev. 2007, 220, 251–269.
Duong, A. D.; Sharma, S.; Peine, K. J.; Gupta, G.; Satoskar, A. R.; Bachelder, E. M.; Wyslouzil, B. E.; Ainslie, K. M. Electrospray encapsulation of toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis. Mol. Pharm. 2013, 10, 1045–1055.
Bachelder, E. M.; Beaudette, T. T.; Broaders, K. E.; Fréchet, J. M. J.; Albrecht, M. T.; Mateczun, A. J.; Ainslie, K. M.; Pesce, J. T.; Keane-Myers, A. M. In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol. Pharm. 2010, 7, 826–835.
Kim, S. Y.; Kim, S.; Kim, J. E.; Lee, S. N.; Shin, I. W.; Shin, H. S.; Jin, S. M.; Noh, Y. W.; Kang, Y. J.; Kim, Y. S. et al. Lyophilizable and multifaceted Toll-like receptor 7/8 agonist-loaded nanoemulsion for the reprogramming of tumor microenvironments and enhanced cancer immunotherapy. ACS Nano 2019, 13, 12671–12686.
Huang, P.; Wang, D. L.; Su, Y.; Huang, W.; Zhou, Y. F.; Cui, D. X.; Zhu, X. Y.; Yan, D. Y. Combination of small molecule prodrug and nanodrug delivery: Amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756.
Zhang, S. Y.; Zou, J.; Elsabahy, M.; Karwa, A.; Li, A.; Moore, D. A.; Dorshow, R. B.; Wooley, K. L. Poly(ethylene oxide)-block-polyphosphester-based paclitaxel conjugates as a platform for ultra-high paclitaxel-loaded multifunctional nanoparticles. Chem. Sci. 2013, 4, 2122–2126.
Zhu, L. J.; Guo, Y. Y.; Qian, Q. H.; Yan, D. Y.; Li, Y. H.; Zhu, X. Y.; Zhang, C. Carrier-free delivery of precise drug-chemogene conjugates for synergistic treatment of drug-resistant cancer. Angew. Chem., Int. Ed. 2020, 59, 17944–17950.
Hu, M.; Zhang, J.; Yu, Y. L.; Tu, K.; Yang, T.; Wang, Y.; Hu, Q.; Kong, L.; Zhang, Z. P. Injectable liquid crystal formation system for reshaping tumor immunosuppressive microenvironment to boost antitumor immunity: Postoperative chemoimmunotherapy. Small 2020, 16, 2004905.
Vinod, N.; Hwang, D.; Azam, S. H.; van Swearingen, A. E. D.; Wayne, E.; Fussell, S. C.; Sokolsky-Papkov, M.; Pecot, C. V.; Kabanov, A. V. High-capacity poly(2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma. Sci. Adv. 2020, 6, eaba5542.
Tomalia, D.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132.
Boas, U.; Heegaard, P. M. H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63.
Hanurry, E. Y.; Mekonnen, T. W.; Andrgie, A. T.; Darge, H. F.; Birhan, Y. S.; Hsu, W. H.; Chou, H. Y.; Cheng, C. C.; Lai, J. Y.; Tsai, H. C. Biotin-decorated PAMAM G4.5 dendrimer nanoparticles to enhance the delivery, anti-proliferative, and apoptotic effects of chemotherapeutic drug in cancer cells. Pharmaceutics 2020, 12, 443.
Zhang, M. E.; Guo, R.; Kéri, M.; Bányai, I.; Zheng, Y.; Cao, M.; Cao, X. Y.; Shi, X. Y. Impact of dendrimer surface functional groups on the release of doxorubicin from dendrimer carriers. J. Phys. Chem. B 2014, 118, 1696–1706.
Cheng, Y. Y.; Li, Y. W.; Wu, Q. L.; Xu, T. W. New insights into the interactions between dendrimers and surfactants by two dimensional NOE NMR spectroscopy. J. Phys. Chem. B 2008, 112, 12674–12680.
Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of dendrimers on solubility of hydrophobic drug molecules. Front. Pharmacol. 2017, 8, 261.
Kulhari, H.; Pooja, D.; Prajapati, S. K.; Chauhan, A. S. Performance evaluation of PAMAM dendrimer based simvastatin formulations. Int. J. Pharm. 2011, 405, 203–209.
Katare, Y. K.; Daya, R. P.; Sookram Gray, C.; Luckham, R. E.; Bhandari, J.; Chauhan, A. S.; Mishra, R. K. Brain targeting of a water insoluble antipsychotic drug haloperidol via the intranasal route using PAMAM dendrimer. Mol. Pharm. 2015, 12, 3380–3388.
Zeng, Y.; Kurokawa, Y.; Win-Shwe, T. T.; Zeng, Q.; Hirano, S.; Zhang, Z. Y.; Sone, H. Effects of PAMAM dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. J. Toxicol. Sci. 2016, 41, 351–370.
Tong, Q. S.; Xu, W.; Huang, Q. Y.; Zhang, Y. R.; Shi, X. X.; Huang, H.; Li, H. J.; Du, J. Z.; Wang, J. Multi-stimuli responsive poly(amidoamine) dendrimers with peripheral N-dialkylaminoethyl carbamate moieties. Polym. Chem. 2019, 10, 656–662.
Hu, J. J.; Cheng, Y. Y.; Ma, Y. R.; Wu, Q. L.; Xu, T. W. Host-guest chemistry and physicochemical properties of the dendrimer-mycophenolic acid complex. J. Phys. Chem. B 2009, 113, 64–74.
D’Emanuele, A.; Attwood, D. Dendrimer-drug interactions. Adv. Drug Deliv. Rev. 2005, 57, 2147–2162.
Wang, H.; Shao, N. N.; Qiao, S. N.; Cheng, Y. Y. Host-guest chemistry of dendrimer-cyclodextrin conjugates: Selective encapsulations of guests within dendrimer or cyclodextrin cavities revealed by NOE NMR techniques. J. Phys. Chem. B 2012, 116, 11217–11224.
Georgoudaki, A. M.; Prokopec, K. E.; Boura, V. F.; Hellqvist, E.; Sohn, S.; Östling, J.; Dahan, R.; Harris, R. A.; Rantalainen, M.; Klevebring, D.; Sund, M. et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016, 15, 2000–2011.
Gardner, A.; Ruffell, B. Dendritic cells and cancer immunity. Trends Immunol. 2016, 37, 855–865.
Acknowledgements
This work was supported by National Key R&D Program of China (No. 2017YFA0205600), Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2017A030306018), National Natural Science Foundation of China (Nos. 51922043 and 31771091), Guangdong Provincial Programs (Nos. 2017ZT07S054 and 2017GC010304), the Science and Technology Program of Guangzhou (No. 201902020018), and Fundamental Research Funds for Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_3510_MOESM1_ESM.pdf
A polyamidoamine (PAMAM) derivative dendrimer with high loading capacity of TLR7/8 agonist for improved cancer immunotherapy
Rights and permissions
About this article
Cite this article
Wu, JS., Li, JX., Shu, N. et al. A polyamidoamine (PAMAM) derivative dendrimer with high loading capacity of TLR7/8 agonist for improved cancer immunotherapy. Nano Res. 15, 510–518 (2022). https://doi.org/10.1007/s12274-021-3510-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3510-0