Abstract
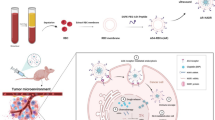
Multidrug resistance (MDR) restricts chemotherapy efficacy due to P-glycoprotein (P-gp) mediated drug efflux, whereas current approaches to suppressing P-gp expression suffer from intrinsic challenges, such as low transfection, high toxicity and poor specificity. Here, hollow ferric-tannic acid complex nanocapsules (HFe-TA), which can be effectively degraded by the reaction with adenosine triphosphate (ATP), are synthesized for the delivery of glucose oxidase (GOx) and doxorubicin (DOX) for tumor treatment. The findings indicate that the intracellular ATP is significantly decreased due to the combined effect of HFe-TA degradation and GOx-mediated glucose consumption. Along with this ATP down-regulation, P-gp expression of tumor cells is suppressed remarkably, which in turn promotes the intracellular accumulation and anticancer efficacy of DOX. In addition, the production of •OH by Fe ions released from HFe-TA is promoted by the by-products of the oxidation of glucose process by GOx. In consequence, HFe-TA nanocapsules loaded with DOX and GOx enable significant inhibition effect to tumors both in vitro and in vivo due to the synergistic effect of cascade reactions. This study has therefore provided an alternative therapeutic platform for effective tumor inhibition with the potential in overcoming intrinsic MDR.

Similar content being viewed by others
References
Jain, R. K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664.
Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237.
Zheng, Y. H.; You, X. R.; Chen, L.; Huang, J.; Wang, L. Y.; Wu, J.; Guan, S. Y. Biotherapeutic nanoparticles of poly(ferulic acid) delivering doxorubicin for cancer therapy. J. Biomed. Nanotechnol. 2019, 15, 1734–1743.
Zhang, X. Q.; Li, L. H.; Liu, Q.; Wang, Y. W.; Yang, J. G.; Qiu, T.; Zhou, G. Co-delivery of rose bengal and doxorubicin nanoparticles for combination photodynamic and chemo-therapy. J. Biomed. Nanotechnol. 2019, 15, 184–195.
Zhu, H. M.; Cao, G. D.; Qiang, C.; Fu, Y. K.; Wu, Y. L.; Li, X.; Han, G. R. Hollow ferric-tannic acid nanocapsules with sustained O2 and ROS induction for synergistic tumor therapy. Biomater. Sci. 2020, 8, 3844–3855.
Song, W.; Das, M.; Xu, Y.; Si, X.; Zhang, Y.; Tang, Z.; Chen, X. Leveraging biomaterials for cancer immunotherapy: Targeting pattern recognition receptors. Mater. Today Nano 2019, 5, 100029.
Xue, W. T.; Di, Z. H.; Zhao, Y.; Zhang, A. P.; Li, L. L. DNA-mediated coordinative assembly of upconversion hetero-nanostructures for targeted dual-modality imaging of cancer cells. Chin. Chem. Lett. 2019, 30, 899–902.
Zhao, J. H.; Chen, J. W.; Ma, S. N.; Liu, Q. Q.; Huang, L. X.; Chen, X. N.; Lou, K. Y.; Wang, W. Recent developments in multimodality fluorescence imaging probes. Acta Pharm. Sin. B 2018, 8, 320–338.
Blanco, E.; Shen, H. F.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951.
Robey, R. W.; Pluchino, K. M.; Hall, M. D.; Fojo, A. T.; Bates, S. E.; Gottesman, M. M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464.
Szakács, G.; Paterson, J. K.; Ludwig, J. A.; Booth-Genthe, C.; Gottesman, M. M. Targeting multidrug resistance in cancer. Nat Rev. Drug Discov. 2006, 5, 219–234.
Chen, C. J.; Chin, J. E.; Ueda, K.; Clark, D. P.; Pastan, I.; Gottesman, M. M.; Roninson, I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 1986, 47, 381–389.
Kirtane, A. R.; Kalscheuer, S. M.; Panyam, J. Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities. Adv. Drug Deliv. Rev. 2013, 65, 1731–1747.
Yang, H.; Shen, X.; Yan, J.; Xie, X. X.; Chen, Z. Y.; Li, T. T.; Li, S.; Qin, X.; Wu, C. H.; Liu, Y. Y. Charge-reversal-functionalized PLGA nanobubbles as theranostic agents for ultrasonic-imaging-guided combination therapy. Biomater. Sci. 2018, 6, 2426–2439.
Wang, C. L.; Guan, W. C.; Peng, J. L.; Chen, Y. T.; Xu, G. X.; Dou, H. J. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020, 103, 247–258.
Zhou, Y. C.; Huang, F. T.; Yang, Y.; Wang, P. L.; Zhang, Z.; Tang, Y. N.; Shen, Y. Q.; Wang, K. Paraptosis-inducing nanomedicine overcomes cancer drug resistance for a potent cancer therapy. Small 2018, 14, 1702446.
Wang, T. T.; Wang, D. G.; Liu, J. P.; Feng, B.; Zhou, F. Y.; Zhang, H. W.; Zhou, L.; Yin, Q.; Zhang, Z. W.; Cao, Z. L. et al. Acidity-triggered ligand-presenting nanoparticles to overcome sequential drug delivery barriers to tumors. Nano Lett. 2017, 17, 5429–5436.
Yao, C.; Wang, P. Y.; Li, X. M.; Hu, X. Y.; Hou, J. L.; Wang, L. Y.; Zhang, F. Near-infrared-triggered azobenzene-liposome/upconversion nanoparticle hybrid vesicles for remotely controlled drug delivery to overcome cancer multidrug resistance. Adv. Mater. 2016, 28, 9341–9348.
Sosnik, A. Reversal of multidrug resistance by the inhibition of ATP-binding cassette pumps employing “generally recognized as safe” (GRAS) nanopharmaceuticals: A review. Adv. Drug Del. Rev. 2013, 65, 1828–1851.
Heiden, M. G. V.; Cantley, L. C.; Thompson, C. B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033.
Wang, H. B.; Li, Y.; Zhang, M. Z.; Wu, D.; Shen, Y. Q.; Tang, G. P.; Ping, Y. Redox-activatable ATP-depleting micelles with dual modulation characteristics for multidrug-resistant cancer therapy. Adv. Healthc. Mater. 2017, 6, 1601293.
Wang, H.; Gao, Z.; Liu, X. Y.; Agarwal, P.; Zhao, S. T.; Conroy, D. W.; Ji, G.; Yu, J. H.; Jaroniec, C. P.; Liu, Z. G. et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 562.
Leist, M.; Single, B.; Castoldi, A. F.; Kühnle, S.; Nicotera, P. Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997, 185, 1481–1486.
Mo, R.; Jiang, T. Y.; DiSanto, R.; Tai, W. Y.; Gu, Z. ATP-triggered anticancer drug delivery. Nat. Commun. 2014, 5, 3364.
Lu, Y.; Aimetti, A. A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2017, 2, 16075.
Mo, R.; Jiang, T. Y.; Gu, Z. Enhanced anticancer efficacy by ATP-mediated liposomal drug delivery. Angew. Chem., Int. Ed. 2014, 126, 5925–5930.
Zhang, L.; Wan, S. S.; Li, C. X.; Xu, L.; Cheng, H.; Zhang, X. Z. An adenosine triphosphate-responsive autocatalytic fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 2018, 18, 7609–7618.
Song, X. R.; Li, S. H.; Dai, J. Y.; Song, L.; Huang, G. M.; Lin, R. H.; Li, J.; Liu, G.; Yang, H. H. Polyphenol-inspired facile construction of smart assemblies for ATP- and pH-responsive tumor MR/optical imaging and photothermal therapy. Small 2017, 13, 1603997.
Xu, C. F.; Liu, Y.; Shen, S.; Zhu, Y. H.; Wang, J. Targeting glucose uptake with siRNA-based nanomedicine for cancer therapy. Biomaterials 2015, 51, 1–11.
Liu, Y.; Cao, Y. Y.; Zhang, W. H.; Bergmeier, S.; Qian, Y. R.; Akbar, H.; Colvin, R.; Ding, J.; Tong, L. Y.; Wu, S. Y. et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 1672–1682.
Chen, W. H.; Luo, G. F.; Lei, Q.; Hong, S.; Qiu, W. X.; Liu, L. H.; Cheng, S. X.; Zhang, X. Z. Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. ACS Nano 2017, 11, 1419–1431.
Zhou, J.; Li, M. H.; Hou, Y. H.; Luo, Z.; Chen, Q. F.; Cao, H. X.; Huo, R. L.; Xue, C. C.; Sutrisno, L.; Hao, L. et al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. ACS Nano 2018, 12, 2858–2872.
Gao, G.; Jiang, Y. W.; Guo, Y. X.; Jia, H. R.; Cheng, X. T.; Deng, Y.; Yu, X. W.; Zhu, Y. X.; Guo, H. Y.; Sun, W. et al. Enzyme-mediated tumor starvation and phototherapy enhance mild-temperature photothermal therapy. Adv. Funct. Mater. 2020, 30, 1909391.
Hu, J. J.; Liu, M. D.; Gao, F.; Chen, Y.; Peng, S. Y.; Li, Z. H.; Cheng, H.; Zhang, X. Z. Photo-controlled liquid metal nanoparticle-enzyme for starvation/photothermal therapy of tumor by win-win cooperation. Biomaterials 2019, 217, 119303.
Sánchez-Laínez, J.; Zornoza, B.; Friebe, S.; Caro, J.; Cao, S.; Sabetghadam, A.; Seoane, B.; Gascon, J.; Kapteijn, F.; Le Guillouzer, C. et al. Influence of ZIF-8 particle size in the performance of polybenzimidazole mixed matrix membranes for pre-combustion CO2 capture and its validation through interlaboratory test. J. Membrane Sci. 2016, 515, 45–53.
Hu, M.; Ju, Y.; Liang, K.; Suma, T.; Cui, J. W.; Caruso, F. Void engineering in metal-organic frameworks via synergistic etching and surface functionalization. Adv. Funct. Mater. 2016, 26, 5827–5834.
Fang, C.; Yan, P. J.; Ren, Z. H.; Wang, Y. F.; Cai, X. J.; Li, X.; Han, G. R. Multifunctional MoO2-ICG nanoplatform for 808 nm-mediated synergetic photodynamic/photothermal therapy. Appl. Mater. Today 2019, 15, 472–481.
Yu, C. J.; Wu, S. M.; Tseng, W. L. Magnetite nanoparticle-induced fluorescence quenching of adenosine triphosphate-BODIPY conjugates: Application to adenosine triphosphate and pyrophosphate sensing. Anal. Chem. 2013, 85, 8559–8565.
Lu, Z. J.; Gao, J. Y.; Fang, C.; Zhou, Y.; Li, X.; Han, G. R. Porous Pt nanospheres incorporated with GOx to enable synergistic oxygen-inductive starvation/electrodynamic tumor therapy. Adv. Sci., in press, DOI: https://doi.org/10.1002/advs.202001223.
Kuang, Y. Y.; Balakrishnan, K.; Gandhi, V.; Peng, X. H. Hydrogen peroxide inducible DNA cross-linking agents: Targeted anticancer prodrugs. J. Am. Chem. Soc. 2011, 133, 19278–19281.
Verrax, J.; Dejeans, N.; Sid, B.; Glorieux, C.; Calderon, P. B. Intracellular ATP levels determine cell death fate of cancer cells exposed to both standard and redox chemotherapeutic agents. Biochem. Pharmacol. 2011, 82, 1540–1548.
Mao, Q. C.; Unadkat, J. D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport-an update. AAPS J. 2015, 17, 65–82.
Zheng, W. P.; Li, M. H.; Lin, Y. X.; Zhan, X. Encapsulation of verapamil and doxorubicin by MPEG-PLA to reverse drug resistance in ovarian cancer. Biomed. Pharmacother. 2018, 108, 565–573.
Gomes, L. C.; Di Benedetto, G.; Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011, 13, 589–598.
Weinberg, S. E.; Sena, L. A.; Chandel, N. S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51672247 and 51902288), Provincial Key research program of Zhejiang Province (No. 2020C04005), ‘111’ Program funded by Education Ministry of China and Sate Bureau of Foreign Experts Affairs (No. B16043), China Postdoctoral Science Foundation (No. 2018M640555), Fundamental Research Funds for the Central Universities and ZJU-Hangzhou Global Scientific and Technological Innovation Center.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Zhu, H., Cao, G., Fu, Y. et al. ATP-responsive hollow nanocapsules for DOX/GOx delivery to enable tumor inhibition with suppressed P-glycoprotein. Nano Res. 14, 222–231 (2021). https://doi.org/10.1007/s12274-020-3071-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3071-7