Abstract
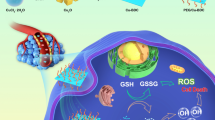
Exploring alternative biomedical use of traditional drugs in different disease models is highly important as it can reduce the cost of drug development and overcome several critical issues of traditional chemodrugs such as low chemotherapeutic efficiency, severe side effect, and drug resistance. Disulfiram (DSF), a clinically approved alcohol-aversion drug, was recently demonstrated to feature tumor-growth suppression effect along with the co-administration of Cu2+ species, but direct Cu2+ administration mode might cause severe toxicity originating from low Cu2+ accumulation into the tumor and nonspecific Cu2+ distribution-induced cytotoxicity. Based on the intriguing drug-delivery performance of nanoscale metal-organic frameworks (MOFs), we herein construct HKUST nMOFs as the Cu2+ self-supplying nanocarriers for efficient delivery of the DSF drug. The mildly acidic condition of tumor microenvironment initially triggered the release of Cu ions from HKUST nMOFs, which further reacted with the encapsulated DSF to form toxic Cu(DDTC)2 (activation) for tumor chemotherapy. Especially, during the Cu(DDTC)2 complexation, Cu+ species were formed concomitantly, triggering the intratumoral nanocatalytic therapy for the generation of reactive oxygen species to synergistically destroying the tumor cells/tissue. As a result, synergetic tumor-responsive chemotherapy and nanocatalytic therapy are enabled by DSF@HKUST nanodrugs, as demonstrated by the dominant anticancer efficacy with satisfied biocompatibility both in vitro and in vivo. The present work offers a sophisticated strategy for tumor-responsive nontoxic-to-toxic therapeutic with high biocompatibility.

Similar content being viewed by others
References
Xue, J. W.; Zhao, Z. K.; Zhang, L.; Xue, L. J.; Shen, S. Y.; Wen, Y. J.; Wei, Z. Y.; Wang, L.; Kong, L. Y.; Sun, H. B. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700.
Ma, P. A.; Xiao, H. H.; Yu, C.; Liu, J. H.; Cheng, Z. Y.; Song, H. Q.; Zhang, X. Y.; Li, C. X.; Wang, J. Q.; Gu, Z. et al. Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett. 2017, 17, 928–937.
Feng, L. L.; Xie, R.; Wang, C. Q.; Gai, S. L.; He, F.; Yang, D.; Yang, P. P.; Lin, J. Magnetic targeting, tumor microenvironment-responsive intelligent nanocatalysts for enhanced tumor ablation. ACS Nano 2018, 12, 11000–11012.
Wang, J.; Luo, C.; Shan, C. L.; You, Q. C.; Lu, J. Y.; Elf, S.; Zhou, Y.; Wen, Y.; Vinkenborg, J. L.; Fan, J. et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem. 2015, 7, 968–979.
Xing, R. R.; Zou, Q. L.; Yuan, C. Q.; Zhao, L. Y.; Chang, R.; Yan, X. H. Self-assembling endogenous biliverdin as a versatile near-infrared photothermal nanoagent for cancer theranostics. Adv. Mater. 2019, 31, 1900822.
Holohan, C.; Van Schaeybroeck, S.; Longley, D. B.; Johnston, P. G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726.
Cao, H. Q.; Wang, Y. X.; He, X. Y.; Zhang, Z. W.; Yin, Q.; Chen, Y.; Yu, H. J.; Huang, Y. Z.; Chen, L. L.; Xu, M. H. et al. Codelivery of sorafenib and curcumin by directed self-assembled nanoparticles enhances therapeutic effect on hepatocellular carcinoma. Mol. Pharm. 2015, 12, 922–931.
Zheng, G. R.; Zhao, R. R.; Xu, A. X.; Shen, Z. C.; Chen, X.; Shao, J. W. Co-delivery of sorafenib and SiVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur. J. Pharm. Sci. 2018, 111, 492–502.
Liang, P. P.; Huang, X. Y.; Wang, Y.; Chen, D. P.; Ou, C. J.; Zhang, Q.; Shao, J. J.; Huang, W.; Dong, X. C. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano 2018, 12, 11446–11457.
Tian, J. W.; Zhou, J. F.; Shen, Z.; Ding, L.; Yu, J. S.; Ju, H. X. A pH-activatable and aniline-substituted photosensitizer for near-infrared cancer theranostics. Chem. Sci. 2015, 6, 5969–5977.
Valko, M.; Rhodes, C. J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40.
Zhang, C.; Chen, W. H.; Liu, L. H.; Qiu, W. X.; Yu, W. Y.; Zhang, X. Z. An O2 self-supplementing and reactive-oxygen-species-circulating amplified nanoplatform via H2O/H2O2 splitting for tumor imaging and photodynamic therapy. Adv. Funct. Mater. 2017, 27, 1700626.
Qu, J.; Zhao, X.; Ma, P. X.; Guo, B. L. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180.
Tseng, S. J.; Kempson, I. M.; Huang, K. Y.; Li, H. J.; Fa, Y. C.; Ho, Y. C.; Liao, Z. X.; Yang, P. C. Targeting tumor microenvironment by bioreduction-activated nanoparticles for light-triggered virotherapy. ACS Nano 2018, 12, 9894–9902.
Lin, L. S.; Huang, T.; Song, J. B.; Ou, X. Y.; Wang, Z. T.; Deng, H. Z.; Tian, R.; Liu, Y. J.; Wang, J. F.; Liu, Y. et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019, 141, 9937–9945.
Cvek, B. Nonprofit drugs as the salvation of the world’s healthcare systems: The case of antabuse (disulfiram). Drug Discov. Today 2012, 17, 409–412.
Duan, X. P.; Xiao, J. S.; Yin, Q.; Zhang, Z. W.; Yu, H. J.; Mao, S. R.; Li, Y. P. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano 2013, 7, 5858–5869.
Tao, X. G.; Gou, J. X.; Zhang, Q. Y.; Tan, X. Y.; Ren, T. Y.; Yao, Q.; Tian, B.; Kou, L. F.; Zhang, L.; Tang, X. Synergistic breast tumor cell killing achieved by intracellular co-delivery of doxorubicin and disulfiram via core-shell-corona nanoparticles. Biomater. Sci. 2018, 6, 1869–1881.
Song, W. T.; Tang, Z. H.; Shen, N.; Yu, H. Y.; Jia, Y. J.; Zhang, D. W.; Jiang, J.; He, C. L.; Tian, H. Y.; Chen, X. S. Combining disulfiram and poly(L-glutamic acid)-cisplatin conjugates for combating cisplatin resistance. J. Control. Release 2016, 231, 94–102.
Zhao, P. F.; Yin, W. M.; Wu, A. H.; Tang, Y. S.; Wang, J. Y.; Pan, Z. Z.; Lin, T. T.; Zhang, M.; Chen, B. F.; Duan, Y. F. et al. Dual-targeting to cancer cells and M2 macrophages via biomimetic delivery of mannosylated albumin nanoparticles for drug-resistant cancer therapy. Adv. Funct. Mater. 2017, 27, 1700403.
Bakthavatsalam, S.; Sleeper, M. L.; Dharani, A.; George, D. J.; Zhang, T.; Franz, K. J. Leveraging γ-glutamyl transferase to direct cytotoxicity of copper dithiocarbamates against prostate cancer cells. Angew. Chem., Int. Ed. 2018, 57, 12780–12784.
Xu, L. Y.; Xu, J. L.; Zhu, J. W.; Yao, Z. J.; Yu, N.; Deng, W.; Wang, Y.; Lin, B. L. Universal anticancer Cu(DTC)2 discriminates between thiols and Zinc (II) thiolates oxidatively. Angew. Chem., Int. Ed. 2019, 131, 6131–6134.
Loo, T. W.; Bartlett, M. C.; Clarke, D. M. Disulfiram metabolites permanently inactivate the human multidrug resistance P-glycoprotein. Mol. Pharm. 2004, 1, 426–433.
Yip, N. C.; Fombon, I. S.; Liu, P.; Brown, S.; Kannappan, V.; Armesilla, A. L.; Xu, B.; Cassidy, J.; Darling, J. L.; Wang, W. Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br. J. Cancer 2011, 104, 1564–1574.
Skrott, Z.; Mistrik, M.; Andersen, K. K.; Friis, S.; Majera, D.; Gursky, J.; Ozdian, T.; Bartkova, J.; Turi, Z.; Moudry, P. et al. Alcohol-abuse drug disulfiram targets cancer via P97 segregase adaptor NPL4. Nature 2017, 552, 194–199.
He, H. C.; Markoutsa, E.; Li, J.; Xu, P. S. Repurposing disulfiram for cancer therapy via targeted nanotechnology through enhanced tumor mass penetration and disassembly. Acta Biomater. 2018, 68, 113–124.
Grubman, A.; White, A. R. Copper as a key regulator of cell signalling pathways. Expert Rev. Mol. Med. 2014, 16, e11.
Lu, K. D.; Aung, T.; Guo, N. N.; Weichselbaum, R.; Lin, W. B. Nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634.
Zhu, W.; Xiang, G. L.; Shang, J.; Guo, J. M.; Motevalli, B.; Durfee, P.; Agola, J. O.; Coker, E. N.; Brinker, C. J. Versatile surface functionalization of metal-organic frameworks through direct metal coordination with a phenolic lipid enables diverse applications. Adv. Funct. Mater. 2018, 28, 1705274.
Lan, G. X.; Ni, K. Y.; Xu, Z. W.; Veroneau, S. S.; Song, Y.; Lin, W. B. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673.
Zhang, Y.; Wang, F. M.; Liu, C. Q.; Wang, Z. Z.; Kang, L. H.; Huang, Y. Y.; Dong, K.; Ren, J. S.; Qu, X. G. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano 2018, 12, 651–661.
Liang, K.; Ricco, R.; Doherty, C. M.; Styles, M. J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A. J.; Doonan, C. J. et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240.
Xiao, J. S.; Zhu, Y. X.; Huddleston, S.; Li, P.; Xiao, B. X.; Farha, O. K.; Ameer, G. A. Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano 2018, 12, 1023–1032.
Xiao, J. S.; Chen, S. Y.; Yi, J.; Zhang, H. F.; Ameer, G. A. A cooperative copper metal-organic framework-hydrogel system improves wound healing in diabetes. Adv. Funct. Mater. 2017, 27, 1604872.
Sun, K. K.; Li, L.; Yu, X. L.; Liu, L.; Meng, Q. T.; Wang, F.; Zhang, R. Functionalization of mixed ligand metal-organic frameworks as the transport vehicles for drugs. J. Colloid Interface Sci. 2017, 486, 128–135.
Wang, X. G.; Cheng, Q.; Yu, Y.; Zhang, X. Z. Controlled nucleation and controlled growth for size predicable synthesis of nanoscale metal-organic frameworks (MOFs): A general and scalable approach. Angew. Chem., Int. Ed. 2018, 57, 7836–7840.
Huo, M. F.; Wang, L. Y.; Wang, Y. W.; Chen, Y.; Shi, J. L. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano 2019, 13, 2643–2653.
Zhang, C. Y.; Yan, L.; Wang, X.; Dong, X. H.; Zhou, R. Y.; Gu, Z. J.; Zhao, Y. L. Tumor microenvironment-responsive Cu2(OH)PO4 nanocrystals for selective and controllable radiosentization via the X-ray-triggered fenton-like reaction. Nano Lett. 2019, 19, 1749–1757.
Lewis, D. J.; Deshmukh, P.; Tedstone, A. A.; Tuna, F.; O’Brien, P. On the interaction of copper(II) with disulfiram. Chem. Commun. 2014, 50, 13334–13337.
Wu, W. C.; Yu, L. D.; Jiang, Q. Z.; Huo, M. F.; Lin, H.; Wang, L. Y.; Chen, Y.; Shi, J. L. Enhanced tumor-specific disulfiram chemotherapy by in situ Cu2+ chelation-initiated nontoxicity-to-toxicity transition. J. Am. Chem. Soc. 2019, 141, 11531–11539.
Mao, D.; Wu, W. B.; Ji, S. D.; Chen, C.; Hu, F.; Kong, D. L.; Ding, D.; Liu, B. Chemiluminescence-guided cancer therapy using a chemiexcited photosensitizer. Chem 2017, 3, 991–1007.
Zhen, X.; Zhang, C. W.; Xie, C.; Miao, Q. Q.; Lim, K. L.; Pu, K. Y. Intraparticle energy level alignment of semiconducting polymer nanoparticles to amplify chemiluminescence for ultrasensitive in vivo imaging of reactive oxygen species. ACS Nano 2016, 10, 6400–6409.
Acknowledgements
We greatly acknowledge the financial support from the National Key R&D Program of China (Nos. 2016YFA0203700 and 2016YFC1101201), the National Natural Science Foundation of China (Nos. 31771026, 81771984, and 51672303), Excellent Young Scientist Foundation of NSFC (No. 51722211), Program of Shanghai Subject Chief Scientist (No. 18XD1404300), and International Collaboration Project of Chinese Academy of Sciences (No. GJHZ2072).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Chen, H., Li, X., Huo, M. et al. Tumor-responsive copper-activated disulfiram for synergetic nanocatalytic tumor therapy. Nano Res. 14, 205–211 (2021). https://doi.org/10.1007/s12274-020-3069-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3069-1