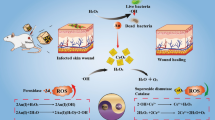
Abstract
Insufficient angiogenesis in the chronic wound of the diabetic is one of the most important causes that making the wound unable to heal itself. In this work, a cobalt-based metal–organic framework (ZIF-67) was introduced as a carrier for loading a pro-angiogenic small molecular drug (dimethyloxalylglycine, DMOG). To achieve a long-term angiogenic therapy on the diabetic wound beds, a dual cooperative controllable release system has been designed by incorporating the drug-loaded ZIF-67 nanoparticles into the micro-patterned PLLA/Gelatin nanofibrous scaffolds. The results showed that DMOG was incorporated into ZIF-67 with a high loading ratio (359.12 mg/g), and the drug-loaded ZIF-67 nanoparticles were well embedded in the circular patterned scaffold. Notably, the DMOG as well as Co ions could continuously release from the scaffold for more than 15 days. The in vitro studies showed that the released Co ions and DMOG from the micropatterned nanofibrous scaffolds could synergistically promote the proliferation, migration and tube formation of the human umbilical vein endothelial cells (HUVECs) by inducing a hypoxia response and upregulating the expression of angiogenesis-related genes such as HIF-1α, VEGF and e-NOS. Furthermore, the in vivo results demonstrated that the composite scaffolds could significantly enhance angiogenesis, collagen deposition and eliminate inflammation in the diabetes wounds. These results indicate that the cobalt-based metal–organic framework as a dual cooperative controllable release system provides a new strategy for enhancing angiogenesis and promoting diabetic wound healing.

Similar content being viewed by others
References
Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest.2007, 117, 1219–1222.
Armstrong, D. G.; Boulton, A. J. M.; Bus, S. A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med.2017, 376, 2367–2375.
Moura, L. I. F.; Dias, A. M. A.; Carvalho, E.; De Sousa, H. C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater.2013, 9, 7093–7114.
Zhang, Q. K.; Oh, J. H.; Park, C. H.; Baek, J. H.; Ryoo, H. M.; Woo, K. M. Effects of dimethyloxalylglycine-embedded poly(ε-caprolactone) fiber meshes on wound healing in diabetic rats. ACS Appl. Mater. Interfaces2017, 9, 7950–7963.
Ren, X. Z.; Han, Y. M.; Wang, J.; Jiang, Y. Q.; Yi, Z. F.; Xu, H.; Ke, Q. F. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater.2018, 70, 140–153.
Li, J.; Wang, X. X.; Zhao, G. X.; Chen, C. L.; Chai, Z. F.; Alsaedi, A.; Hayat, T.; Wang, X. K. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev.2018, 47, 2322–2356.
Xuan, W. M.; Zhu, C. F.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev.2012, 41, 1677–1695.
Bloch, E. D.; Queen, W. L.; Krishna, R.; Zadrozny, J. M.; Brown, C. M.; Long, J. R. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science2012, 335, 1606–1610.
Wang, Z. B.; Knebel, A.; Grosjean, S.; Wagner, D.; Bräse, S.; Wöll, C.; Caro, J.; Heinke, L. Tunable molecular separation by nanoporous membranes. Nat. Commun.2016, 7, 13872.
Peters, A. W.; Li, Z. Y.; Farha, O. K.; Hupp, J. T. Toward inexpensive photocatalytic hydrogen evolution: A nickel sulfide catalyst supported on a high-stability metal-organic framework. ACS Appl. Mater. Interfaces2016, 8, 20675–20681.
Zhuang, Y. X.; Zhang, X. D.; Chen, Q. M.; Li, S. Q.; Cao, H. Y.; Huang, Y. M. Co3O4/CuO hollow nanocage hybrids with high oxidase-like activity for biosensing of dopamine. Mater. Sci. Eng. C.2019, 94, 858–866.
Wu, M. X.; Yang, Y. W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater.2017, 29, 1606134.
Li, H. Y.; Lv, N. N.; Li, X.; Liu, B. T.; Feng, J.; Ren, X. H.; Guo, T.; Chen, D. W.; Stoddart, J. F.; Gref, R. et al. Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery. Nanoscale2017, 9, 7454–7463.
Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J. M.; Marshall, R. J.; Sastre, B.; Del Pozo, V.; Fairen-Jimenez, D.; Forgan, R. S. Surface-functionalization of Zr-fumarate MOF for selective cytotoxicity and immune system compatibility in nanoscale drug delivery. ACS Appl. Mater. Interfaces2018, 10, 31146–31157.
Zhang, Y.; Sun, P. P.; Zhang, L.; Wang, Z. Z.; Wang, F. M.; Dong, K.; Liu, Z.; Ren, J. S.; Qu, X. G. Silver-infused porphyrinic metal–organic framework: Surface-adaptive, on-demand nanoplatform for synergistic bacteria killing and wound disinfection. Adv. Funct. Mater.2019, 29, 1808594.
Liu, X. P.; Yan, Z. Q.; Zhang, Y.; Liu, Z. W.; Sun, Y. H.; Ren, J. S.; Qu, X. G. Two-dimensional metal-organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano2019, 13, 5222–5230.
Xiao, J. S.; Zhu, Y. X.; Huddleston, S.; Li, P.; Xiao, B. X.; Farha, O. K.; Ameer, G. A. Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano2018, 12, 1023–1032.
Xiao, J. S.; Chen, S. Y.; Yi, J.; Zhang, H. F.; Ameer, G. A. A cooperative copper metal–organic framework-hydrogel system improves wound healing in diabetes. Adv. Fun. Mater.2017, 27, 1604872.
Wang, L. L.; Zhu, H. L.; Shi, Y.; Ge, Y.; Feng, X. M.; Liu, R. Q.; Li, Y.; Ma, Y. W.; Wang, L. H. Novel catalytic micromotor of porous zeolitic imidazolate framework-67 for precise drug delivery. Nanoscale2018, 10, 11384–11391.
Simonsen, L. O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total Environ.2012, 432, 210–215.
Pacary, E.; Legros, H.; Valable, S.; Duchatelle, P.; Lecocq, M.; Petit, E.; Nicole, O.; Bernaudin, M. Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J. Cell Sci.2006, 119, 2667–2678.
Park, K. S.; Ni, Z.; Côté, A. P.; Choi, J. Y.; Huang, R. D.; Uribe-Romo, F. J.; Chae, H. K.; O’Keeffe, M.; Yaghi, O. M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA2006, 103, 10186–10191.
Zheng, M.; Liu, S.; Guan, X. G.; Xie, Z. G. One-step synthesis of nanoscale zeolitic imidazolate frameworks with high curcumin loading for treatment of cervical cancer. ACS Appl. Mater. Interfaces2015, 7, 22181–22187.
Gao, S. T.; Jin, Y.; Ge, K.; Li, Z. H.; Liu, H. F.; Dai, X. Y.; Zhang, Y. H.; Chen, S. Z.; Liang, X. J.; Zhang, J. C. Self-supply of O2 and H2O2 by a nanocatalytic medicine to enhance combined chemo/chemodynamic therapy. Adv. Sci. 2019, 6, 1902137.
Zhang, J. L.; Cai, Y. N.; Liu, K. X. Extremely effective boron removal from water by stable metal organic framework ZIF-67. Ind. Eng. Chem. Res.2019, 58, 4199–4207.
Hu, Y. W.; Song, X. D.; Zheng, Q. L.; Wang, J. N.; Pei, J. F. Zeolitic imidazolate framework-67 for shape stabilization and enhanced thermal stability of paraffin-based phase change materials. RSC Adv.2019, 9, 9962–9967.
Lin, K. Y. A.; Wu, C. H. Efficient adsorptive removal of toxic amaranth dye from water using a zeolitic imidazolate framework. Water Environ. Res.2018, 90, 1947–1955.
Yuan, Q.; Bleiziffer, O.; Boos, A. M.; Sun, J. M.; Brandl, A.; Beier, J. P.; Arkudas, A.; Schmitz, M.; Kneser, U.; Horch, R. E. PHDs inhibitor DMOG promotes the vascularization process in the AV loop by HIF-1α up-regulation and the preliminary discussion on its kinetics in rat. BMC Biotechnol.2014, 14, 112.
Jayarama Reddy, V.; Radhakrishnan, S.; Ravichandran, R.; Mukherjee, S.; Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen.2013, 21, 1–16.
Xu, H.; Lv, F.; Zhang, Y. L.; Yi, Z. F.; Ke, Q. F.; Wu, C. T.; Liu, M. Y.; Chang, J. Hierarchically micro-patterned nanofibrous scaffolds with a nanosized bio-glass surface for accelerating wound healing. Nanoscale2015, 7, 18446–18452.
Lv, F.; Wang, J.; Xu, P.; Han, Y. M.; Ma, H. S.; Xu, H.; Chen, S. J.; Chang, J.; Ke, Q. F.; Liu, M. Y. et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater.2017, 60, 128–143.
Yin, H. Y.; Wang, J.; Gu, Z. Q.; Feng, W. H.; Gao, M. C.; Wu, Y.; Zheng, H.; He, X. M.; Mo, X. M. Evaluation of the potential of kartogenin encapsulated poly(L-lactic acid-co-caprolactone)/collagen nanofibers for tracheal cartilage regeneration. J. Biomater. Appl.2017, 32, 331–341.
Lee, C. H.; Hsieh, M. J.; Chang, S. H.; Lin, Y. H.; Liu, S. J.; Lin, T. Y.; Hung, K. C.; Pang, J. H. S.; Juang, J. H. Enhancement of diabetic wound repair using biodegradable nanofibrous metformineluting membranes: In vitro and in vivo. ACS Appl. Mater. Interfaces2014, 6, 3979–3986.
Xu, H.; Li, H. Y.; Ke, Q. F.; Chang, J. An anisotropically and heterogeneously aligned patterned electrospun scaffold with tailored mechanical property and improved bioactivity for vascular tissue engineering. ACS Appl. Mater. Interfaces2015, 7, 8706–8718.
Lei, Y. F.; Zouani, O. F.; Rami, L.; Chanseau, C.; Durrieu, M. C. Modulation of lumen formation by microgeometrical bioactive cues and migration mode of actin machinery. Small2013, 9, 1086–1095.
Xie, J. W.; Liu, W. Y.; MacEwan, M. R.; Yeh, Y. C.; Thomopoulos, S.; Xia, Y. N. Nanofiber membranes with controllable microwells and structural cues and their use in forming cell microarrays and neuronal networks. Small2011, 7, 293–297.
Jiang, Z.; Li, Z. P.; Qin, Z. H.; Sun, H. Y.; Jiao, X. L.; Chen, D. R. LDH nanocages synthesized with MOF templates and their high performance as supercapacitors. Nanoscale2013, 5, 11770–11775.
Lü, Y. Y.; Wang, Y. T.; Li, H. L.; Lin, Y.; Jiang, Z. Y.; Xie, Z. X.; Kuang, Q.; Zheng, L. S. MOF-derived porous Co/C nanocomposites with excellent electromagnetic wave absorption properties. ACS Appl. Mater. Interfaces2015, 7, 13604–13611.
Li, H. Y.; Chang, J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater.2013, 9, 5379–5389.
Wu, Y. L.; Quan, Y. C.; Liu, Y. Q.; Liu, K. W.; Li, H. Q.; Jiang, Z. W.; Zhang, T.; Lei, H.; Radek, K. A.; Li, D. Q. et al. Hyperglycaemia inhibits REG3A expression to exacerbate TLR3-mediated skin inflammation in diabetes. Nat. Commun.2016, 7, 13393.
Ammar, M.; Jiang, S.; Ji, S. F. Heteropoly acid encapsulated into zeolite imidazolate framework (ZIF-67) cage as an efficient heterogeneous catalyst for Friedel-Crafts acylation. J. Solid State Chem.2016, 233, 303–310.
Chun, N. Y.; Kim, S. N.; Choi, Y. S.; Choy, Y. B. PCN-223 as a drug carrier for potential treatment of colorectal cancer. J. Ind. Eng. Chem.2020, 84, 290–296.
Yang, Y.; Xia, T.; Chen, F.; Wei, W.; Liu, C. Y.; He, S. H.; Li, X. H. Electrospun fibers with plasmid bFGF polyplex loadings promote skin wound healing in diabetic rats. Mol. Pharmaceutics2012, 9, 48–58.
Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials2007, 28, 3074–3082.
Shi, Q. Y.; Luo, X.; Huang, Z. Q.; Midgley, A. C.; Wang, B.; Liu, R. H.; Zhi, D. K.; Wei, T. T.; Zhou, X.; Qiao, M. Q. et al. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater.2019, 86, 465–479.
Kim, H. H.; Lee, S. E.; Chung, W. J.; Choi, Y.; Kwack, K.; Kim, S. W.; Kim, M. S.; Park, H.; Lee, Z. H. Stabilization of hypoxia-inducible factor-1α is involved in the hypoxic stimuli-induced expression of vascular endothelial growth factor in osteoblastic cells. Cytokine2002, 17, 14–27.
Okonkwo, U. A.; Dipietro, L. A. Diabetes and wound angiogenesis. Int. J. Mol. Sci.2017, 18, 1419.
Wu, C. T.; Zhou, Y. H.; Fan, W.; Han, P. P.; Chang, J.; Yuen, J.; Zhang, M. L.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials2012, 33, 2076–2085.
Namiki, A.; Brogi, E.; Kearney, M.; Kim, E. A.; Wu, T. G.; Couffinhal, T.; Varticovski, L.; Isner, J. M. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J. Biol. Chem.1995, 270, 31189–31195.
Fan, W.; Crawford, R.; Xiao, Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials2010, 31, 3580–3589.
Gao, W.; Sun, L.; Fu, X.; Lin, Z.; Xie, W.; Zhang, W.; Zhao, F.; Chen, X. Enhanced diabetic wound healing by electrospun core-sheath fibers loaded with dimethyloxalylglycine. J. Mater. Chem. B2018, 6, 277–288.
Groenman, F. A.; Rutter, M.; Wang, J. X.; Caniggia, I.; Tibboel, D.; Post, M. Effect of chemical stabilizers of hypoxia-inducible factors on early lung development. Am. J. Physiol. Lung Cell. Mol. Physiol.2007, 293, L557–L567.
Valarmathi, M. T.; Davis, J. M.; Yost, M. J.; Goodwin, R. L.; Potts, J. D. A three-dimensional model of vasculogenesis. Biomaterials2009, 30, 1098–1112.
McClure, M. J.; Wolfe, P. S.; Simpson, D. G.; Sell, S. A.; Bowlin, G. L. The use of air-flow impedance to control fiber deposition patterns during electrospinning. Biomaterials2012, 33, 771–779.
Da Silva, I. R.; Da Tiveron, L. C. R. C.; Da Silva, M. V.; Peixoto, A. B.; Carneiro, C. A. X.; Dos Reis, M. A.; Furtado, P. C.; Rodrigues, B. R.; Rodrigues, V.; Rodrigues, D. B. R. In situ cytokine expression and morphometric evaluation of total collagen and collagens Type I and Type III in Keloid scars. Mediators Inflamm.2017, 2017, 6573802.
Wells, A.; Nuschke, A.; Yates, C. C. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol.2016, 49, 25–36.
Lai, H. J.; Kuan, C. H.; Wu, H. C.; Tsai, J. C.; Chen, T. M.; Hsieh, D. J.; Wang, T. W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater.2014, 10, 4156–4166.
Van Putte, L.; De Schrijver, S.; Moortgat, P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: A systematic review. Scars, Burns Heal.2016, 2, 2059513116676828.
Garwood, C. S.; Steinberg, J. S.; Kim, P. J. Bioengineered alternative tissues in diabetic wound healing. Clin. Podiatr. Med. Surg.2015, 32, 121–133.
Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol.2000, 182, 311–322.
Dalby, M. J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M. O.; Herzyk, P.; Wilkinson, C. D. W.; Oreffo, R. O. C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater.2007, 6, 997–1003.
Eming, S. A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med.2014, 6, 265sr6.
Babensee, J. E.; Anderson, J. M.; McIntire, L. V.; Mikos, A. G. Host response to tissue engineered devices. Adv. Drug Deliv. Rev.1998, 33, 111–139.
Scholz, C. C.; Cavadas, M. A. S.; Tambuwala, M. M.; Hams, E.; Rodríguez, J.; Von Kriegsheim, A.; Cotter, P.; Bruning, U.; Fallon, P. G.; Cheong, A. et al. Regulation of IL-1β-induced NF-κB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc. Natl. Acad. Sci. USA2013, 110, 18490–18495.
Bandarra D.; Biddlestone J.; Mudie S.; Müller, H. A. J.; Rocha, S. HIF-1α restricts NF-κB-dependent gene expression to control innate immunity signals. Dis. Models Mech.2015, 8, 169–181.
Wang C.; Sun H. R.; Song Y.; Ma Z. S.; Zhang G.; Gu X. H.; Zhao L. Pterostilbene attenuates inflammation in rat heart subjected to ischemia-reperfusion: Role of TLR4/NF-κB signaling pathway. Int. J. Clin. Exp. Med.2015, 8, 1737–1746.
Acknowledgements
This work was supported by the Natural Science Foundation of Shanghai (No. 19ZR1437800).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2020_2846_MOESM1_ESM.pdf
Cobalt-based metal–organic framework as a dual cooperative controllable release system for accelerating diabetic wound healing
Rights and permissions
About this article
Cite this article
Li, J., Lv, F., Li, J. et al. Cobalt-based metal–organic framework as a dual cooperative controllable release system for accelerating diabetic wound healing. Nano Res. 13, 2268–2279 (2020). https://doi.org/10.1007/s12274-020-2846-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2846-1