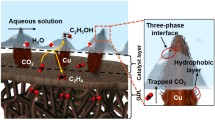
Abstract
Electrocatalytic CO2 reduction is a promising way to mitigate the urgent energy and environmental issues, but how to increase the selectivity for desired product among multiple competing reaction pathways remains a bottleneck. Here, we demonstrate that engineering the gas–liquid–solid contact interface on the electrode surface could tailor the selectivity of CO2 reduction and meanwhile suppress H2 production through regulated reaction kinetics. Specifically, polytetrafluoroethylene (PTFE) was utilized to modify a Cu nanoarray electrode as an example, which is able to change the electrode surface from aerophobic to aerophilic state. The enriched nano-tunnels of the Cu nanoarray electrode can facilitate CO2 transportation and pin gaseous products on the electrode surface. The latter is believed to be the reason that boosts the Faradaic efficiency of liquid products by 67% and limits the H2 production to less than half of before. This interface engineering strategy also lowered H2O (proton) affinity, therefore promoting CO and HCOOH production. Engineering the electrode contact interface controls the reaction kinetics and the selectivity of products, which should be inspiring for other electrochemical reactions.

Similar content being viewed by others
References
Schreier, M.; Héroguel, F.; Steier, L.; Ahmad, S.; Luterbacher, J. S.; Mayer, M. T.; Luo, J. S.; Grätzel, M. Solar conversion of CO2 to CO using earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy 2017, 2, 17087.
Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G. S.; Kimmel, Y. C.; Chen, J. G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242.
Qiao, J. L.; Liu, Y. Y.; Hong, F.; Zhang, J. J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675.
Lei, F. C.; Liu, W.; Sun, Y. F.; Xu, J. Q.; Liu, K. T.; Liang, L.; Yao, T.; Pan, B. C.; Wei, S. Q.; Xie, Y. Metallic tin quantum sheets confined in graphene toward high-efficiency carbon dioxide electroreduction. Nat. Commun. 2016, 7, 12697.
Gao, S.; Sun, Z. T.; Liu, W.; Jiao, X. C.; Zu, X. L.; Hu, Q. T.; Sun, Y. F.; Yao, T.; Zhang, W. H.; Wei, S. Q. et al. Atomic layer confined vacancies for atomic-level insights into carbon dioxide electroreduction. Nat. Commun. 2017, 8, 14503.
Chen, Y. H.; Kanan, M. W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts. J. Am. Chem. Soc. 2012, 134, 1986–1989.
Li, F. W.; Chen, L.; Knowles, G. P.; MacFarlane, D. R.; Zhang, J. Hierarchical mesoporous SnO2 nanosheets on carbon cloth: A robust and flexible electrocatalyst for CO2 reduction with high efficiency and selectivity. Angew. Chem., Int. Ed. 2017, 56, 505–509.
Jiang, K.; Wang, H.; Cai, W. B.; Wang, H. T. Li electrochemical tuning of metal oxide for highly selective CO2 reduction. ACS Nano 2017, 11, 6451–6458.
Zhang, S.; Kang, P.; Meyer, T. J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 2014, 136, 1734–1737.
Kim, C.; Jeon, H. S.; Eom, T.; Jee, M. S.; Kim, H.; Friend, C. M.; Min, B. K.; Hwang, Y. J. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850.
Liu, S. B.; Tao, H. B.; Zeng, L.; Liu, Q.; Xu, Z. H.; Liu, Q. X.; Luo, J. L. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163.
Luc, W.; Collins, C.; Wang, S. W.; Xin, H. L.; He, K.; Kang, Y. J.; Jiao, F. Ag-Sn bimetallic catalyst with a core-shell structure for CO2 reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893.
Gao, D. F.; Zhang, Y.; Zhou, Z. W.; Cai, F.; Zhao, X. F.; Huang, W. G.; Li, Y. S.; Zhu, J. F.; Liu, P.; Yang, F. et al. Enhancing CO2 electroreduction with the metal-oxide interface. J. Am. Chem. Soc. 2017, 139, 5652–5655.
Kumar, B.; Atla, V.; Brian, J. P.; Kumari, S.; Nguyen, T. Q.; Sunkara, M.; Spurgeon, J. M. Reduced SnO2 porous nanowires with a high density of grain boundaries as catalysts for efficient electrochemical CO2-into- HCOOH conversion. Angew. Chem., Int. Ed. 2017, 56, 3645–3649.
Huo, S. J.; Weng, Z.; Wu, Z. S.; Zhong, Y. R.; Wu, Y. S.; Fang, J. H.; Wang, H. L. Coupled metal/oxide catalysts with tunable product selectivity for electrocatalytic CO2 reduction. ACS Appl. Mater. Interfaces 2017, 9, 28519–28526.
Varela, A. S.; Ju, W.; Reier, T.; Strasser, P. Tuning the catalytic activity and selectivity of Cu for CO2 electroreduction in the presence of halides. ACS Catal. 2016, 6, 2136–2144.
Hahn, C.; Hatsukade, T.; Kim, Y. G.; Vailionis, A.; Baricuatro, J. H.; Higgins, D. C.; Nitopi, S. A.; Soriaga, M. P.; Jaramillo, T. F. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl. Acad. Sci. USA 2017, 114, 5918–5923.
Weng, Z.; Zhang, X.; Wu, Y. S.; Huo, S. J.; Jiang, J. B.; Liu, W.; He, G. J.; Liang, Y. Y.; Wang, H. L. Self-cleaning catalyst electrodes for stabilized CO2 reduction to hydrocarbons. Angew. Chem., Int. Ed. 2017, 56, 13135–13139.
Weng, Z.; Jiang, J. B.; Wu, Y. S.; Wu, Z. S.; Guo, X. T.; Materna, K. L.; Liu, W.; Batista, V. S.; Brudvig, G. W.; Wang, H. L. Electrochemical CO2 reduction to hydrocarbons on a heterogeneous molecular Cu catalyst in aqueous solution. J. Am. Chem. Soc. 2016, 138, 8076–8079.
Li, Q.; Fu, J. J.; Zhu, W. L.; Chen, Z. Z.; Shen, B.; Wu, L. H.; Xi, Z.; Wang, T. Y.; Lu, G.; Zhu, J. J. et al. Tuning Sn-catalysis for electrochemical reduction of CO2 to CO via the core/shell Cu/SnO2 structure. J. Am. Chem. Soc. 2017, 139, 4290–4293.
Li, Y. F.; Cui, F.; Ross, M. B.; Kim, D.; Sun, Y. C.; Yang, P. D. Structuresensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 2017, 17, 1312–1317.
Dai, L.; Qin, Q.; Wang, P.; Zhao, X. J.; Hu, C. Y.; Liu, P. X.; Qin, R. X.; Chen, M.; Ou, D. H.; Xu, C. F. et al. Ultrastable atomic copper nanosheets for selective electrochemical reduction of carbon dioxide. Sci. Adv. 2017, 3, e1701069.
Ma, S. C.; Sadakiyo, M.; Heima, M.; Luo, R.; Haasch, R. T.; Gold, J. I.; Yamauchi, M.; Kenis, P. J. A. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu-Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 2016, 139, 47–50.
Sarfraz, S.; Garcia-Esparza, A. T.; Jedidi, A.; Cavallo, L.; Takanabe, K. Cu-Sn bimetallic catalyst for selective aqueous electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851.
Li, C. W.; Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234.
Verdaguer-Casadevall, A.; Li, C. W.; Johansson, T. P.; Scott, S. B.; McKeown, J. T.; Kumar, M.; Stephens, I. E. L.; Kanan, M. W.; Chorkendorff, I. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 2015, 137, 9808–9811.
Li, P. S.; Xie, Q. X.; Zheng, L. R.; Feng, G.; Li, Y. J.; Cai, Z.; Bi, Y. M.; Li, Y. P.; Kuang, Y.; Sun, X. M. et al. Topotactic reduction of layered double hydroxides for atomically thick two-dimensional non-noble-metal alloy. Nano Res. 2017, 10, 2988–2997.
Weng, Z.; Wu, Y. S.; Wang, M. Y.; Jiang, J. B.; Yang, K.; Huo, S. J.; Wang, X. F.; Ma, Q.; Brudvig, G. W.; Batista, V. S. et al. Active sites of coppercomplex catalytic materials for electrochemical carbon dioxide reduction. Nat. Commun. 2018, 9, 415.
Cai, Z.; Zhou, D. J.; Wang, M. Y.; Bak, S. M.; Wu, Y. S.; Wu, Z. S.; Tian, Y.; Xiong, X. Y.; Li, Y. P.; Liu, W. et al. Introducing Fe2+ into nickel-iron layered double hydroxide: Local structure modulated water oxidation activity. Angew. Chem., Int. Ed. 2018, 130, 9536–9540.
Zhou, D. J.; Cai, Z.; Lei, X. D.; Tian, W. L.; Bi, Y. M.; Jia, Y.; Han, N. N.; Gao, T. F.; Zhang, Q.; Kuang, Y. et al. NiCoFe-layered double hydroxides/N-doped graphene oxide array colloid composite as an efficient bifunctional catalyst for oxygen electrocatalytic reactions. Adv. Energy Mater. 2018, 8, 1701905.
Han, N. N.; Yang, K. R.; Lu, Z. Y.; Li, Y. J.; Xu, W. W.; Gao, T. F.; Cai, Z.; Zhang, Y.; Batista, V. S.; Liu, W. et al. Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 2018, 9, 924.
Cai, Z.; Bi, Y. M.; Hu, E. Y.; Liu, W.; Dwarica, N.; Tian, Y.; Li, X. L.; Kuang, Y.; Li, Y. P.; Yang, X. Q. et al. Single-crystalline ultrathin Co3O4 nanosheets with massive vacancy defects for enhanced electrocatalysis. Adv. Energy Mater. 2018, 8, 1701694.
Zhou, Y.; Silva, J. L.; Woods, J. M.; Pondick, J. V.; Feng, Q. L.; Liang, Z. X.; Liu, W.; Lin, L.; Deng, B. C.; Brena, B. et al. Revealing the contribution of individual factors to hydrogen evolution reaction catalytic activity. Adv. Mater. 2018, 30, 1706076.
Ma, M.; Djanashvili, K.; Smith, W. A. Controllable hydrocarbon formation from the electrochemical reduction of CO2 over Cu nanowire arrays. Angew. Chem., Int. Ed. 2016, 55, 6680–6684.
Lu, Z. Y.; Xu, W. W.; Ma, J.; Li, Y. J.; Sun, X. M.; Jiang, L. Superaerophilic carbon-nanotube-array electrode for high-performance oxygen reduction reaction. Adv. Mater. 2016, 28, 7155–7161.
Lu, Z. Y.; Zhu, W.; Yu, X. Y.; Zhang, H. C.; Li, Y. J.; Sun, X. M.; Wang, X. W.; Wang, H.; Wang, J. M.; Luo, J. et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014, 26, 2683–2687.
Tian, X. L.; Verho, T.; Ras, R. H. A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142–143.
Morales-Guio, C. G.; Stern, L. A.; Hu, X. L. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569.
Zhu, S. Q.; Jiang, B.; Cai, W. B.; Shao, M. H. Direct observation on reaction intermediates and the role of bicarbonate anions in CO2 electrochemical reduction reaction on Cu surfaces. J. Am. Chem. Soc. 2017, 139, 15664–15667.
Schouten, K. J. P.; Kwon, Y.; Van der Ham, C. J. M.; Qin, Z.; Koper, M. T. M. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2011, 2, 1902–1909.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China (Nos. 2016YFC0801302 and 2016YFF0204402), the Program for Changjiang Scholars and Innovative Research Team in the University, the Fundamental Research Funds for the Central Universities, and the longterm subsidy mechanism from the Ministry of Finance and the Ministry of Education of PRC. Y. X. Z. thanks the National Natural Science Foundation of China (No. 61701543) for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2018_2221_MOESM1_ESM.pdf
Selectivity regulation of CO2 electroreduction through contact interface engineering on superwetting Cu nanoarray electrodes
Rights and permissions
About this article
Cite this article
Cai, Z., Zhang, Y., Zhao, Y. et al. Selectivity regulation of CO2 electroreduction through contact interface engineering on superwetting Cu nanoarray electrodes. Nano Res. 12, 345–349 (2019). https://doi.org/10.1007/s12274-018-2221-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2221-7