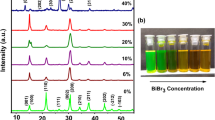
Abstract
In this work, the pseudohalide thiocyanate has been demonstrated as a promising alternative to the halide anion to engineer optoelectronic properties of inorganic/organic hybrid perovskites because it exhibits better chemical stability than the halide anion. Previous reports have suggested that the ionic radii and electronegativity of SCN− is close to that of I−; the SCN− doped CH3NH3PbI3 exhibited similar optical properties as pure CH3NH3PbI3. Consequently, it was expected that doping of CsPbBr3 perovskite with SCN− would result in band gap narrowing. Interestingly, the photoluminescent all-inorganic CsPbBr3 perovskite nanocrystals exhibit an abnormal blue shift in optical properties and improvement of the crystallinity when successfully doped by SCN−. Combined experimental and theoretical investigations revealed that doping of the CsPbBr3 perovskite with the rod-like SCN− anion introduced disorder in the crystal lattice, leading to its expansion, and impacted the electronic structure of the perovskite with band gap broadening.

Similar content being viewed by others
References
Huang, X. Q.; Zhao, Z. P.; Cao, L.; Chen, Y.; Zhu, E. B.; Lin, Z. Y.; Li, M. F.; Yan, A. M.; Zettl, A.; Wang, Y. M. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234.
Burschka, J.; Pellet, N.; Moon, S. J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M. K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319.
Jeon, N. J.; Noh, J. H.; Yang, W. S.; Kim, Y. C.; Ryu, S.; Seo, J.; Seok, S. I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480.
Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M. I.; Grotevent, M. J.; Kovalenko, M. V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640.
Protesescu, L.; Yakunin, S.; Bodnarchuk, M. I.; Krieg, F.; Caputo, R.; Hendon, C. H.; Yang, R. X.; Walsh, A.; Kovalenko, M. V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696.
Li, X. M.; Wu, Y.; Zhang, S. L.; Cai, B.; Gu, Y.; Song, J. Z.; Zeng, H. B. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445.
Stoumpos, C. C.; Malliakas, C. D.; Peters, J. A.; Liu, Z. F.; Sebastian, M.; Im, J.; Chasapis, T. C.; Wibowo, A. C.; Chung, D. Y.; Freeman, A. J. et al. Crystal growth of the perovskite semiconductor CsPbBr3: A new material for high-energy radiation detection. Cryst. Growth Des. 2013, 13, 2722–2727.
Song, J. Z.; Li, J. H.; Li, X. M.; Xu, L. M.; Dong, Y. H.; Zeng, H. B. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167.
Wang, Y.; Li, X. M.; Song, J. Z.; Xiao, L.; Zeng, H. B.; Sun, H. D. All-inorganic colloidal perovskite quantum dots: A new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 27, 7101–7108.
Akkerman, Q. A.; Motti, S. G.; Srimath Kandada, A. R.; Mosconi, E.; D’Innocenzo, V.; Bertoni, G.; Marras, S.; Kamino, B. A.; Miranda, L.; De Angelis, F. et al. Solution synthesis approach to colloidal cesium lead halide perovskite nanoplatelets with monolayer-level thickness control. J. Am. Chem. Soc. 2016, 138, 1010–1016.
Chen, Y. H.; Chen, T.; Dai, L. M. Layer-by-layer growth of CH3NH3PbI3–xClx for highly efficient planar heterojunction perovskite solar cells. Adv. Mater. 2015, 27, 1053–1059.
Akkerman, Q. A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281.
Park, B. W.; Philippe, B.; Zhang, X. L.; Rensmo, H.; Boschloo, G.; Johansson, E. M. J. Bismuth based hybrid perovskites A3Bi2I9 (A: methylammonium or cesium) for solar cell application. Adv. Mater. 2015, 27, 6806–6813.
Li, Q. L.; Lu, W. X.; Wan, N.; Ding, S. N. Tuning optical properties of perovskite nanocrystals by supermolecular mercapto-β-cyclodextrin. Chem. Commun. 2016, 52, 12342–12345.
Stoumpos, C. C.; Malliakas, C. D.; Kanatzidis, M. G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and nearinfrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038.
Sun, S. B.; Yuan, D.; Xu, Y.; Wang, A. F.; Deng, Z. T. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano 2016, 10, 3648–3657.
Gonzalez-Carrero, S.; Galian, R. E.; Pérez-Prieto, J. Organic-inorganic and all-inorganic lead halide nanoparticles [Invited]. Opt. Express 2016, 24, A285–A301.
Zhang, F.; Zhong, H. Z.; Chen, C.; Wu, X. G.; Hu, X. M.; Huang, H. L.; Han, J. B.; Zou, B. S.; Dong, Y. P. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology. ACS Nano 2015, 9, 4533–4542.
Sutton, R. J.; Eperon, G. E.; Miranda, L.; Parrott, E. S.; Kamino, B. A.; Patel, J. B.; Hörantner, M. T.; Johnston, M. B.; Haghighirad, A. A.; Moore, D. T. et al. Bandgaptunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv. Energy Mater. 2016, 6, 1502458.
Chen, Y. N.; Li, B. B.; Huang, W.; Gao, D. Q.; Liang, Z. Q. Efficient and reproducible CH3NH3PbI3–x(SCN)x perovskite based planar solar cells. Chem. Commun. 2015, 51, 11997–11999.
Tai, Q. D.; You, P.; Sang, H. Q.; Liu, Z. K.; Hu, C. L.; Chan, H. L. W.; Yan, F. Efficient and stable perovskite solar cells prepared in ambient air irrespective of the humidity. Nat. Commun. 2016, 7, 11105.
Chiang, Y. H.; Li, M. H.; Cheng, H. M.; Shen, P. S.; Chen, P. Mixed cation thiocyanate-based pseudohalide perovskite solar cells with high efficiency and stability. ACS Appl. Mater. Interfaces 2017, 9, 2403–2409.
Halder, A.; Chulliyil, R.; Subbiah, A. S.; Khan, T.; Chattoraj, S.; Chowdhury, A.; Sarkar, S. K. Pseudohalide (SCN–)-doped MAPbI3 perovskites: A few surprises. J. Phys. Chem. Lett. 2015, 6, 3483–3489.
Xiao, Z. W.; Meng, W. W.; Saparov, B.; Duan, H. S.; Wang, C. L.; Feng, C. B.; Liao, W. Q.; Ke, W. J.; Zhao, D. W.; Wang, J. B. et al. Photovoltaic properties of two-dimensional (CH3NH3)2Pb(SCN)2I2 perovskite: A combined experimental and density functional theory study. J. Phys. Chem. Lett. 2016, 7, 1213–1218.
Jiang, Q. L.; Rebollar, D.; Gong, J.; Piacentino, E. L.; Zheng, C.; Xu, T. Pseudohalide-induced moisture tolerance in perovskite CH3NH3Pb(SCN)2I thin films. Angew. Chem., Int. Ed. 2015, 54, 7617–7620.
Daub, M.; Hillebrecht, H. Synthesis, single-crystal structure and characterization of (CH3NH3)2Pb(SCN)2I2. Angew. Chem., Int. Ed. 2015, 54, 11016–11017.
Tang, G.; Yang, C.; Stroppa, A.; Fang, D. N.; Hong, J. W. Revealing the role of thiocyanate anion in layered hybrid halide perovskite (CH3NH3)2Pb(SCN)2I2. J. Chem. Phys. 2017, 146, 224702.
Wei, S.; Yang, Y. C.; Kang, X. J.; Wang, L.; Huang, L. J.; Pan, D. C. Room-temperature and gram-scale synthesis of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals with 50%–85% photoluminescence quantum yields. Chem. Commun. 2016, 52, 7265–7268.
Zhao, Y. X.; Zhu, K. Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 2016, 45, 655–689.
Li, Z.; Yang, M. J.; Park, J.-S.; Wei, S.-H.; Berry, J. J.; Zhu, K. Stabilizing perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016, 28, 284–292.
Chen, Y.; He, M. H.; Peng, J. J.; Sun, Y.; Liang, Z. Q. Structure and growth control of organic-inorganic halide perovskites for optoelectronics: From polycrystalline films to single crystals. Adv. Sci. 2016, 3, 1500392.
Dastidar, S.; Egger, D. A.; Tan, L. Z.; Cromer, S. B.; Dillon, A. D.; Liu, S.; Kronik, L.; Rappe, A. M.; Fafarman, A. T. High chloride doping levels stabilize the perovskite phase of cesium lead iodide. Nano Lett. 2016, 16, 3563–3570.
Sadhanala, A.; Ahmad, S.; Zhao, B. D.; Giesbrecht, N.; Pearce, P. M.; Deschler, F.; Hoye, R. L. Z.; Gödel, K. C.; Bein, T.; Docampo, P. et al. Blue-green color tunable solution processable organolead chloride-bromide mixed halide perovskites for optoelectronic applications. Nano Lett. 2015, 15, 6095–6101.
Hoke, E. T.; Slotcavage, D. J.; Dohner, E. R.; Bowring, A. R.; Karunadasa, H. I.; McGehee, M. D. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 2015, 6, 613–617.
Kim, Y.; Yassitepe, E.; Voznyy, O.; Comin, R.; Walters, G.; Gong, X. W.; Kanjanaboos, P.; Nogueira, A. F.; Sargent, E. H. Efficient luminescence from perovskite quantum dot solids. ACS Appl. Mater. Interfaces 2015, 7, 25007–25013.
De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J. C.; Van Driessche, I.; Kovalenko, M. V.; Hens, Z. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano 2016, 10, 2071–2081.
Bekenstein, Y.; Koscher, B. A.; Eaton, S. W.; Yang, P. D.; Alivisatos, A. P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011.
Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y. F.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942.
Yang, D. W.; Lv, J.; Zhao, X. G.; Xu, Q. L.; Fu, Y. H.; Zhan, Y. Q.; Zunger, A.; Zhang, L. J. Functionality-directed screening of Pb-free hybrid organic–inorganic perovskites with desired intrinsic photovoltaic functionalities. Chem. Mater. 2017, 29, 524–538.
Boyd, R. J.; Boyd, S. L. Group electronegativities from the bond critical point model. J. Am. Chem. Soc. 1992, 114, 1652–1655.
Leonard, G. W.; Smith, M. E.; Hume, D. N. Thiocyanate complexes of lead and thallium in solution. J. Phys. Chem. 1956, 60, 1493–1495.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (Nos. 21475021, 21427807, 61722403, 11404131, and 11674121), the Natural Science Foundation of Jiangsu Province (No. BK20141331), the Fundamental Research Funds for the Central Universities, Program for JLU Science and Technology Innovative Research Team, the Special Fund for Talent Exploitation in Jilin Province of China, Jiangsu provincial financial support of fundamental conditions and science and technology for people’s livelihood for Jiangsu key laboratory of advanced metallic materials (No. BM2007204).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lou, Y., Niu, Y., Yang, D. et al. Rod-shaped thiocyanate-induced abnormal band gap broadening in SCN− doped CsPbBr3 perovskite nanocrystals. Nano Res. 11, 2715–2723 (2018). https://doi.org/10.1007/s12274-017-1901-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1901-z