Abstract
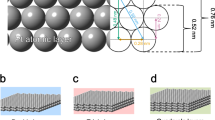
To fully realize the commercial viability of Pt in fuel cells, the usage of scarce Pt must be reduced while the activity and durability in O2 reduction reaction (ORR) must be enhanced. Here we report a metallic stack design achieving these goals for ORR, based on atomically precise materials synthesis. Au@Pd@Pt nanostructures with atomically thin Pt shells and high-index surfaces form an excellent platform for integrating the effects of electronic structures, surface facets, and substrate stabilization to boost ORR performance. Au@Pd@Pt trisoctahedrons (TOH) achieve mass activity 6.1 times higher than that of commercial Pt/C and dramatically enhanced durability beyond 1.0 V vs. a reversible hydrogen electrode in oxidation potential. Meanwhile, Pt comprises only 3.2% of the nanostructures. To further improve the ORR activity and demonstrate the versatility of our strategy, we implement the same design in PtNi alloy electrocatalysts. The Au@Pd@PtNi TOHs exhibit mass activity 14.3 times higher than that of commercial Pt/C as well as excellent durability. This work demonstrates an alternative strategy for fabricating high-performance and low-cost catalysts, and highlights the importance of simultaneous surface and interfacial engineering with atomic precision in designing catalysts.

Similar content being viewed by others
References
He, W. H.; Wang Y.; Jiang, C. H.; Lu, L. H. Structural effects of a carbon matrix in non-precious metal O2-reduction electrocatalysts. Chem. Soc. Rev. 2016, 45, 2396–2409.
Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66.
Shao, M. H.; Chang, Q. W.; Dodelet, J. P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657.
Chen, C.; Kang, Y. J.; Huo, Z. Y.; Zhu, Z. W.; Huang, W. Y.; Xin, H. L.; Snyder, J. D.; Li, D. G.; Herron, J. A.; Mavrikakis, M. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343.
Zhang, L.; Roling, L. T.; Wang, X.; Vara, M.; Chi, M. F.; Liu, J. Y.; Choi, S. I.; Park, J.; Herron, J. A.; Xie, Z. X. et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412–416.
Huang, X. Q.; Zhao, Z. P.; Cao, L. P.; Chen, Y.; Zhu, E. B.; Lin, Z. Y.; Li, M. F.; Yan, A. M.; Zettl, A.; Wang, Y. M. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234.
Guo, S. J.; Li, D. G.; Zhu, H. Y.; Zhang, S.; Markovic, N. M.; Stamenkovic, V. R.; Sun, S. H. FePt and CoPt nanowires as efficient catalysts for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2013, 52, 3465–3468.
USGS. Mineral Commodity Summaries 2016; U.S. Geological Survey: Reston, Virginia, 2016.
Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R. R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222.
Bian, T.; Zhang, H.; Jiang Y. Y.; Jin, C. H.; Wu, J. B.; Yang, H.; Yang, D. R. Epitaxial growth of twinned Au-Pt core-shell star-shaped decahedra as highly durable electrocatalysts. Nano Lett. 2015, 15, 7808–7815.
Bu, L. Z.; Zhang, N.; Guo, S. J.; Zhang, X.; Li, J.; Yao, J. L.; Wu, T.; Lu, G.; Ma, J. Y.; Su, D. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2017, 354, 1410–1414.
Mazumder, V.; Chi, M. F.; More, K. L.; Sun, S. H. Core/shell Pd/FePt nanoparticles as an active and durable catalyst for the oxygen reduction reaction. J. Am. Chem. Soc. 2010, 132, 7848–7849.
Zhang, S.; Hao, Y. Z.; Su, D.; Doan-Nguyen, V. V. T.; Wu, Y. T.; Li, J.; Sun, S. H.; Marray, C. B. Monodisperse core/shell Ni/FePt nanoparticles and their conversion to Ni/Pt to catalyze oxygen reduction. J. Am. Chem. Soc. 2014, 136, 15921–15924.
Wang, J. X.; Inada, H.; Wu, L. J.; Zhu, Y. M.; Choi, Y. M.; Liu, P.; Zhou, W. P.; Adzic, R. R. Oxygen reduction on well-defined core-shell nanocatalysts: Particle size, facet, and Pt shell thickness effects. J. Am. Chem. Soc. 2009, 131, 17298–17302.
Ma, L.; Wang, C. M.; Xia, B. Y.; Mao, K. K.; He, J. W.; Wu, X. J.; Xiong, Y. J.; Lou, X. W. Platinum multicubes prepared by Ni2+-mediated shape evolution exhibit high electrocatalytic activity for oxygen reduction. Angew. Chem., Int. Ed. 2015, 54, 5666–5671.
Xu, X. L.; Zhang, X.; Sun, H.; Yang, Y.; Dai, X. P.; Gao, J. S.; Li, X. Y.; Zhang, P. F.; Wang, H. H.; Yu, N. F. et al. Synthesis of Pt-Ni alloy nanocrystals with high-index facets and enhanced electrocatalytic properties. Angew. Chem., Int. Ed. 2014, 126, 12730–12735.
Greeley, J.; Stephens, I. E. L.; Bondarenko, A. S.; Johansson, T. P.; Hansen, H. A.; Jaramillo, T. F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J. K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556.
Bai, S.; Wang, C. M.; Deng, M. S.; Gong, M.; Bai, Y.; Jiang, J.; Xiong, Y. J. Surface polarization matters: Enhancing the hydrogen-evolution reaction by shrinking Pt shells in Pt-Pd-graphene stack structures. Angew. Chem., Int. Ed. 2014, 53, 12120–12124.
Chen, G. X.; Xu, C. F.; Huang, X. Q.; Ye, J. Y.; Gu, L.; Li, G.; Tang, Z. C.; Wu, B. H.; Yang, H. Y.; Zhao, Z. P. et al. Interfacial electronic effects control the reaction selectivity of platinum catalysts. Nat. Mater. 2016, 15, 564–569.
Hu, J.; Wu, L. J.; Kuttiyiel, K. A.; Goodman, K. R.; Zhang, C. X.; Zhu, Y. M.; Vukmirovic, M. B.; White, M. G.; Sasaki, K.; Adzic, R. R. Increasing stability and activity of core-shell catalysts by preferential segregation of oxide on edges and vertexes: Oxygen reduction on Ti-Au@ Pt/C. J. Am. Chem. Soc. 2016, 138, 9294–9300.
Sasaki, K.; Naohara, H.; Choi, Y.; Cai, Y.; Chen, W. F.; Liu, P.; Adzic, R. R. Highly stable Pt monolayer on PdAu nanoparticle electrocatalysts for the oxygen reduction reaction. Nat. Commun. 2012, 3, 1115.
Kang, Y. J.; Snyder, J.; Chi, M. F.; Li, D. G.; More, K. L.; Markovic, N. M.; Stamenkovic, V. R. Multimetallic core/ interlayer/shell nanostructures as advanced electrocatalysts. Nano Lett. 2014, 14, 6361–6367.
Zhang, J.; Langille, M. R.; Personick, M. L.; Zhang, K.; Li, S. Y.; Mirkin, C. A. Concave cubic gold nanocrystals with high-index facets. J. Am. Chem. Soc. 2010, 132, 14012–14014.
Tan, S. F.; Chee, S. W.; Lin, G. H.; Bosman, M.; Lin, M.; Mirsaidov, U.; Nijhuis, C. A. Real-time imaging of the formation of Au-Ag core-shell nanoparticles. J. Am. Chem. Soc. 2016, 138, 5190–5193.
van der Vliet, D.; Strmcnik, D. S.; Wang, C.; Stamenkovic, V. R.; Markovic, N. M.; Koper, M. T. M. On the importance of correcting for the uncompensated ohmic resistance in model experiments of the oxygen reduction reaction. J. Electrochem. Soc. 2010, 647, 29–34.
Kresse, G.; Furthmiiller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50.
Mortensen, J. J.; Hansen, L. B.; Jacobsen, K. W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 2005, 71, 035109.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Monkhorst, H. J.; Pack, J. D. Special points for Brillouinzone integrations. Phys. Rev. B 1976, 13, 5188–5192.
Rossmeisl, J.; Logadottir, A.; Nørskov, J. K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184.
Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
Ma, Y. Y.; Kuang, Q.; Jiang, Z. Y.; Xie, Z. X.; Huang, R. B.; Zheng, L. S. Synthesis of trisoctahedral gold nanocrystals with exposed high-index facets by a facile chemical method. Angew. Chem., Int. Ed. 2008, 47, 8901–8904.
Niu, W. X.; Chua, Y. A. A.; Zhang, W. Q.; Huang, H. J.; Lu, X. M. Highly symmetric gold nanostars: Crystallographic control and surface-enhanced Raman scattering property. J. Am. Chem. Soc. 2015, 137, 10460–10463.
Langille, M. R.; Personick, M. L.; Zhang, J.; Mirkin, C. A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554.
Fan, F. R.; Liu, D. Y.; Wu, Y. F.; Duan, S.; Xie, Z. X.; Jiang, Z. Y.; Tian, Z. Q. Epitaxial growth of heterogeneous metal nanocrystals: From gold nano-octahedra to palladium and silver nanocubes. J. Am. Chem. Soc. 2008, 130, 6949–6951.
Xie, S. F.; Choi, S. I.; Lu, N.; Roling, L. T.; Herron, J. A.; Zhang, L.; Park, J.; Wang, J. G.; Kim, M. J.; Xie, Z. X. et al. Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 2014, 14, 3570–3576.
Wang, F.; Li, C. H.; Sun, L. D.; Wu, H. S.; Ming, T.; Wang, J. F.; Yu, J. C.; Yan, C. H. Heteroepitaxial growth of high-index-faceted palladium nanoshells and their catalytic performance. J. Am. Chem. Soc. 2011, 133, 1106–1111.
Yu, Y.; Zhang, Q. B.; Liu, B.; Lee, J. Y. Synthesis of nanocrystals with variable high-index Pd facets through the controlled heteroepitaxial growth of trisoctahedral Au templates. J. Am. Chem. Soc. 2010, 132, 18258–18265.
Zhang, Q. F.; Wang, H. Facet-dependent catalytic activities of Au nanoparticles enclosed by high-index facets. ACS Catal. 2014, 4, 4027–4033.
Tao, F.; Grass, M. E.; Zhang, Y.; Butcher, D. R.; Renzas, J. R.; Liu, Z.; Chung, J. Y.; Mun, B. S.; Salmeron, M.; Somorjai, G. A. Reaction-driven restructuring of Rh-Pd and Pt-Pd core-shell nanoparticles. Science 2008, 322, 932–936.
Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G. F.; Ross, P. N.; Lucas, C. A.; Markovic, N. M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497.
Stamenkovic, V. R.; Mun, B. S.; Arenz, M.; Mayrhofer, K. J. J.; Lucas, C. A.; Wang, G. F.; Ross, P. N.; Markovic, N. M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247.
Lindström, R. W.; Korstdottir, K.; Wesselmark, M.; Oyarce, A.; Lagergren, C.; Lindbergh, G. Active area determination of porous Pt electrodes used in polymer electrolyte fuel cells: Temperature and humidity effects. J. Electrochem. Soc. 2010, 157, B1795–B1801.
Lee, S. J.; Mukerjee, S.; McBreen, J.; Rho, Y. W.; Kho, Y. T.; Lee, T. H. Effects of nafion impregnation on performances of PEMFC electrodes. Electrochimica Acta 1998, 43, 3693–3701.
He, D. P.; Jiang, Y. L.; Lv, H. F.; Pan, M.; Mu, S. C. Nitrogen-doped reduced graphene oxide supports for noble metal catalysts with greatly enhanced activity and stability. Appl. Catal. B: Environ. 2013, 132–133, 379–388.
Snyder, J.; Fujita, T.; Chen, M. W.; Erlebacher, J. Oxygen reduction in nanoporous metal-ionic liquid composite electrocatalysts. Nat. Mater. 2010, 9, 904–907.
Bai, S.; Yang, L.; Wang, C. L.; Lin, Y.; Lu, J. L.; Jiang, J.; Xiong, Y. J. Boosting photocatalytic water splitting: Interfacial charge polarization in atomically controlled core-shell cocatalysts. Angew. Chem., Int. Ed. 2015, 54, 14810–14814.
Xia, B. Y.; Wang, B.; Wu, H. B.; Liu, Z. L.; Wang, X.; Lou, X. W. Sandwich-structured TiO2-Pt-graphene ternary hybrid electrocatalysts with high efficiency and stability. J. Mater. Chem. 2012, 22, 16499–16505.
Zhang, J. L.; Vukmirovic, M. B.; Sasaki, K.; Nilekar, A. U.; Mavrikakis, M.; Adzic, R. R. Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics. J. Am. Chem. Soc. 2005, 127, 12480-12481.
Kang, Y. J.; Ye, X. C.; Chen, J.; Cai, Y.; Diaz, R. E.; Adzic, R. R.; Stach, E. A.; Murray, C. B. Design of Pt-Pd binary superlattices exploiting shape effects and synergistic effects for oxygen reduction reactions. J. Am. Chem. Soc. 2013, 135, 42–45.
Duan, Z. Y.; Wang, G. F. Comparison of reaction energetics for oxygen reduction reactions on Pt(100), Pt(111), Pt/Ni(100), and Pt/Ni(111) surfaces: A first-principles study. J. Phys. Chem. C 2013, 117, 6284–6292.
Nilekar, A. U.; Mavrikakis, M. Improved oxygen reduction reactivity of platinum monolayers on transition metal surfaces. Surf. Sci. 2008, 602, 89–94.
Acknowledgements
This work was financially supported in part by the National Key R&D Program of China (No. 2017YFA- 0207301), the National Natural Science Foundation of China (NSFC) (Nos. 21471141, U1532135 and 21573212), CAS Key Research Program of Frontier Sciences (No. QYZDB-SSW-SLH018), CAS Interdisciplinary Innovation Team, Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology (No. 2016FXCX003), Recruitment Program of Global Experts, CAS Hundred Talent Program, and Anhui Provincial Natural Science Foundation (Nos. 1608085QB24 and 1508085MB24). X.W. was supported by the MOST (No. 2016YFA0200602), the National Natural Science Foundation of China (NSFC) (Nos. 21421063, 51172223 and 21573204), Strategic Priority Research Program of CAS (No. XDB01020300), the National Key Basic Research Program (No. 2012CB922001), National Program for Support of Top-notch Young Professional, External Cooperation Program of BIC CAS (No. 211134KYSB20130017), and by USTCSCC, SCCAS, Tianjin, and Shanghai Supercomputer Centers. Depth-dependent XPS experiments were performed at the Photoemission Endstation at the BL10B beamline in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2017_1891_MOESM1_ESM.pdf
Enhanced O2 reduction on atomically thin Pt-based nanoshells by integrating surface facet, interfacial electronic, and substrate stabilization effects
Rights and permissions
About this article
Cite this article
Ye, W., Sun, Z., Wang, C. et al. Enhanced O2 reduction on atomically thin Pt-based nanoshells by integrating surface facet, interfacial electronic, and substrate stabilization effects. Nano Res. 11, 3313–3326 (2018). https://doi.org/10.1007/s12274-017-1891-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1891-x