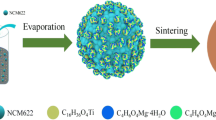
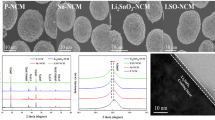
Abstract
Li-rich layered cathode materials have been considered the most promising candidates for large-scale Li-ion batteries due to their low cost and high reversible capacity. However, these materials have many drawbacks that hinder commercialization, such as low initial efficiency and cyclability at elevated temperatures. To overcome these barriers, we propose an efficient and effective surface modification method, in which chemical activation (acid treatment) and LiCoPO4 coating were carried out simultaneously. During the synthesis, the lithium ions were extracted from the lattice, leading to improved Columbic efficiency, and these ions were used for the formation of LiCoPO4. The Ni and Co doped spinel phase was formed at the surface of the host material, which gives rise to the facile pathway for lithium ions. The LiCoPO4 and highly doped spinel on the surface acted as double protection layers that effectively prevented side reactions on the surface at 60 °C. Moreover, the transition metal migration of the modified cathode was weakened, due to the presence of the spinel structure at the surface. Consequently, the newly developed Li-rich cathode material exhibited a high 1st efficiency of 94%, improved capacity retention of 82% during 100 cycles at 60 °C, and superior rate capability of 62% at 12C (1C = 200 mA/g) rate at 24 °C. In addition, the thermal stability of the modified cathode was significantly improved as compared to that of a bare counterpart at 4.6 V, showing a 60% decrease in the total heat generation.

Similar content being viewed by others
References
Choi, N. S.; Chen, Z. H.; Freunberger, S. A.; Ji, X. L.; Sun, Y. K.; Amine, K.; Yushin, G.; Nazar, L. F.; Cho, J.; Bruce, P. G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem., Int. Ed. 2012, 51, 9994–10024.
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Thackeray, M. M.; Wolverton, C.; Isaacs, E. D. Electrical energy storage for transportation-approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863.
Sun, Y. K.; Lee, M. J.; Yoon, C. S.; Hassoun, J.; Amine, K.; Scrosati, B. The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-ion batteries. Adv. Mat. 2012, 24, 1192–1196.
Gu, M.; Belharouak, I.; Genc, A.; Wang, Z. G.; Wang, D. P.; Amine, K.; Gao, F.; Zhou, G. W.; Thevuthasan, S.; Baer, D. R. et al. Conflicting roles of nickel in controlling cathode performance in lithium ion batteries. Nano Lett. 2012, 12, 5186–5191.
Yu, H. J.; Ishikawa, R.; So, Y. G.; Shibata, N.; Kudo, T.; Zhou, H. S.; Ikuhara, Y. Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries. Angew. Chem., Int. Ed. 2013, 52, 5969–5973.
Makimura, Y.; Ohzuku, T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 156–160.
Sun, Y. K.; Myung, S. T.; Park, B. C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320–324.
Cho, Y.; Oh, P.; Cho, J. A new type of protective surface layer for high-capacity Ni-based cathode materials: Nanoscaled surface pillaring layer. Nano Lett. 2013, 13, 1145–1152.
Wu, C. R.; Fang, X. P.; Guo, X. W.; Mao, Y.; Ma, J.; Zhao, C. C.; Wang, Z. X.; Chen, L. Q. Surface modification of Li1.2Mn0.54Co0.13Ni0.13O2 with conducting polypyrrole. J. Power Sources 2013, 231, 44–49.
Yabuuchi, N.; Lu, Y. C.; Mansour, A. N.; Chen, S.; Shao-Horn, Y. The influence of heat-treatment temperature on the cation distribution of LiNi0.5Mn0.5O2 and its rate capability in lithium rechargeable batteries. J. Electrochem. Soc. 2011, 158, A192–A200.
Croy, J. R.; Kim, D.; Balasubramanian, M.; Gallagher, K.; Kang, S. H.; Thackeray, M. M. Countering the voltage decay in high capacity xLi2MnO3·(1–x)LiMO2 electrodes (M = Mn, Ni, Co) for Li+-ion batteries. J. Electrochem. Soc. 2012, 159, A781–A790.
Wang, J.; Yuan, G. X.; Zhang, M. H.; Qiu, B.; Xia, Y. G.; Liu, Z. P. The structure, morphology, and electrochemical properties of Li1+xNi1/6Co1/6Mn4/6O2.25+x/2 (0.1 ≤ x ≤ 0.7) cathode materials. Electrochim. Acta 2012, 66, 61–66.
Jung, Y. S.; Cavanagh, A. S.; Yan, Y. F.; George, S. M.; Manthiram, A. Effects of atomic layer deposition of Al2O3 on the Li[Li0.20Mn0.54Ni0.13Co0.13]O2 cathode for lithium-ion batteries. J. Electrochem. Soc. 2011, 158, A1298–A1302.
Park, M. S.; Lee, J. W.; Choi, W.; Im, D.; Doo, S. G.; Park, K. S. On the surface modifications of high-voltage oxide cathodes for lithium-ion batteries: New insight and significant safety improvement. J. Mater. Chem. 2010, 20, 7208–7213.
Zhang, X. F.; Belharouak, I.; Li, L.; Lei, Y.; Elam, J. W.; Nie, A. M.; Chen, X. Q.; Yassar, R. S.; Axelbaum, R. L. Structural and electrochemical study of Al2O3 and TiO2 coated Li1.2Ni0.13Mn0.54Co0.13O2 cathode material using ALD. Adv. Energy Mater. 2013, 3, 1299–1307.
Kang, S. H.; Thackeray, M. M. Enhancing the rate capability of high capacity xLi2MnO3·(1–x)LiMO2 (M = Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment. Electrochem. Commun. 2009, 11, 748–751.
Qiao, Q. Q.; Zhang, H. Z.; Li, G. R.; Ye, S. H.; Wang, C. W.; Gao, X. P. Surface modification of Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide with Li-Mn-PO4 as the cathode for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 5262–5268.
Kang, S. H.; Johnson, C. S.; Vaughey, J. T.; Amine, K.; Thackeray, M. M. The effects of acid treatment on the electrochemical properties of 0.5Li2MnO3·0.5LiNi0.44Co0.25Mn0.31O2 electrodes in lithium cells. J. Electrochem. Soc. 2006, 153, A1186–A1192.
Xu, G. F.; Li, J. L.; Xue, Q. R.; Ren, X. P.; Yan, G.; Wang, X. D.; Kang, F. Y. Enhanced oxygen reducibility of 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 cathode material with mild acid treatment. J. Power Sources 2014, 248, 894–899.
Kim, J. S.; Johnson, C. S.; Vaughey, J. T.; Thackeray, M. M. Pre-conditioned layered electrodes for lithium batteries. J. Power Sources 2006, 153, 258–264.
Oh, P.; Ko, M.; Myeong, S.; Kim, Y.; Cho, J. A novel surface treatment method and new insight into discharge voltage deterioration for high-performance 0.4Li2MnO3–0.6LiNi1/3Co1/3Mn1/3O2 cathode materials. Adv. Energy Mater. 2014, 4, 1400631, DOI: 10.1002/aenm.201400631.
Oh, P.; Myeong, S.; Cho, W.; Lee, M. J.; Ko, M.; Jeong, H. Y.; Cho, J. Superior long-term energy retention and volumetric energy density for Li-rich cathode materials. Nano Lett. 2014, 14, 5965–5972.
Wu, F.; Li, N.; Su, Y. F.; Zhang, L. J.; Bao, L. Y.; Wang, J.; Chen, L.; Zheng, Y.; Dai, L. Q.; Peng, J. Y. et al. Ultrathin spinel membrane-encapsulated layered lithium-rich cathode material for advanced Li-ion batteries. Nano Lett. 2014, 14, 3550–3555.
Gu, M.; Genc, A.; Belharouak, I.; Wang, D. P.; Amine, K.; Thevuthasan, S.; Baer, D. R.; Zhang, J. G.; Browning, N. D.; Liu, J. et al. Nanoscale phase separation, cation ordering, and surface chemistry in pristine Li1.2Ni0.2Mn0.6O2 for Li-ion batteries. Chem. Mater. 2013, 25, 2319–2326.
Zheng, J. M.; Gu, M.; Genc, A.; Xiao, J.; Xu, P. H.; Chen, X. L.; Zhu, Z. H.; Zhao, W. B.; Pullan, L.; Wang, C. M. et al. Mitigating voltage fade in cathode materials by improving the atomic level uniformity of elemental distribution. Nano Lett. 2014, 14, 2628–2635.
Kim, Y.; Cho, J. Lithium-reactive Co3 (PO4)2 nanoparticle coating on high-capacity LiNi0.8Co0.16Al0.04O2 cathode material for lithium rechargeable batteries. J. Electrochem. Soc. 2007, 154, A495–A499.
Park, M. H.; Noh, M.; Lee, S.; Ko, M.; Chae, S.; Sim, S.; Choi, S.; Kim, H.; Nam, H.; Park, S. et al. Flexible highenergy Li-ion batteries with fast-charging capability. Nano Lett. 2014, 14, 4083–4089.
Lee, M. J.; Lee, S.; Oh, P.; Kim, Y.; Cho, J. High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-ion batteries. Nano Lett. 2014, 14, 993–999.
Huang, R.; Ikuhara, Y. H.; Mizoguchi, T.; Findlay, S. D.; Kuwabara, A.; Fisher, C. A. J.; Moriwake, H.; Oki, H.; Hirayama, T.; Ikuhara, Y. Oxygen-vacancy ordering at surfaces of lithium manganese(III, IV) oxide spinel nanoparticles. Angew. Chem., Int. Ed. 2011, 50, 3053–3057.
Thackeray, M. M.; Kang, S. H.; Johnson, C. S.; Vaughey, J. T.; Benedek, R.; Hackney, S. A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125.
Johnson, C. S.; Li, N.; Vaughey, J. T.; Hackney, S. A.; Thackeray, M. M. Lithium-manganese oxide electrodes with layered-spinel composite structures xLi2MnO3·(1–x)Li1+yMn2–yO4 (0 < x < 1, 0 ≤ y ≤ 0.33) for lithium batteries. Electrochem. Commun. 2005, 7, 528–536.
Wu, F.; Li, N.; Su, Y. F.; Shou, H. F.; Bao, L. Y.; Yang, W.; Zhang, L. J.; An, R.; Chen, S. Spinel/layered heterostructured cathode material for high-capacity and high-rate Li-ion batteries. Adv. Mat. 2013, 25, 3722–3726.
Ohzuku, T.; Takeda, S.; Iwanaga, M. Solid-state redox potentials for Li[Me1/2Mn3/2]O4 (Me: 3d-transition metal) having spinel-framework structures: A series of 5 volt materials for advanced lithium-ion batteries. J. Power Sources 1999, 81–82, 90–94.
Kim, J. H.; Myung, S. T.; Yoon, C. S.; Kang, S. G.; Sun, Y. K. Comparative study of LiNi0.5Mn1.5O4−δ and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: \(Fd\bar 3m\) and P4332. Chem. Mater. 2004, 16, 906–914.
Han, D. W.; Kang, Y. M.; Yin, R. Z.; Song, M. S.; Kwon, H. S. Effects of Fe doping on the electrochemical performance of LiCoPO4/C composites for high power-density cathode materials. Electrochem. Commun. 2009, 11, 137–140.
Rui, X. H.; Zhao, X. X.; Lu, Z. Y.; Tan, H. T.; Sim, D.; Hng, H. H.; Yazami, R.; Lim, T. M.; Yan, Q. Y. Olivine-type nanosheets for lithium ion battery cathodes. ACS Nano 2013, 7, 5637–5646.
Acknowledgements
This work was supported by the IT R&D program of MOTIE/KEIT (Development of Li-rich Cathode and Carbon-free Anode Materials for High Capacity/High Rate Lithium secondary Batteries, No. 10046309).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
12274_2017_1662_MOESM1_ESM.pdf
Simultaneous surface modification method for 0.4Li2MnO3- 0.6LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries: Acid treatment and LiCoPO4 coating
Rights and permissions
About this article
Cite this article
Lee, MJ., Lho, E., Oh, P. et al. Simultaneous surface modification method for 0.4Li2MnO3-0.6LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries: Acid treatment and LiCoPO4 coating. Nano Res. 10, 4210–4220 (2017). https://doi.org/10.1007/s12274-017-1662-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1662-8