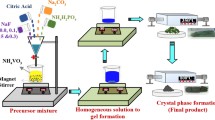
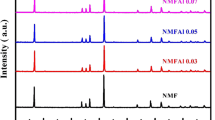
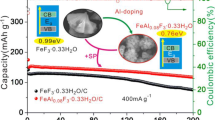
Abstract
Na-ion batteries (NIBs) are considered one of the most attractive alternatives for Li-ion batteries (LIBs) because of the natural abundance of Na and the similarities between the NIB technology and the well-established LIB technology. However, the discovery of high-performance electrode materials remains a key factor in the success of NIBs. Herein, we propose a new type of cathode material for NIBs based on a nanocomposite of an alkali metal fluoride (NaF) and a transition metal fluoride (FeF2). Although neither of these components is electrochemically active with Na, the nanoscale mixture of the two can deliver a reversible capacity of ∼125 mAh/g in the voltage range of 1.2–4.8 V vs. Na/Na+ via an Fe2+/Fe3+ redox couple. X-ray absorption spectroscopy reveals that the reversible Na storage is aided by the F–ions due to the decomposition of NaF, which are absorbed on the surface of FeF2, promoting the redox reaction of Fe and triggering the gradual transformation of the mother structure (FeF2) into a new (FeF3-like) host structure for the Na ions. This unique Na-ion storage phenomenon, which is reported for the first time, will introduce an avenue for designing novel cathode materials for NIBs.

Similar content being viewed by others
References
Shakoor, R. A.; Seo, D.-H.; Kim, H.; Park, Y.-U.; Kim, J.; Kim, S.-W.; Gwon, H.; Lee, S.; Kang, K. A combined first principles and experimental study on Na3V2(PO4)2F3 for rechargeable Na batteries. J. Mater. Chem. 2012, 22, 20535–20541.
Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K. B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901.
Pan, H. L.; Hu, Y.-S.; Chen, L. Q. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360.
Kim, H.; Kim, H.; Ding, Z.; Lee, M. H.; Lim, K.; Yoon, G.; Kang, K. Recent progress in electrode materials for sodiumion batteries. Adv. Energy Mater. 2016, 6, 1600943.
Kim, S. W.; Seo, D. H.; Ma, X. H.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721.
Slater, M. D.; Kim, D.; Lee, E.; Johnson, C. S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958.
Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682.
Kim, D.; Lee, E.; Slater, M.; Lu, W. Q.; Rood, S.; Johnson, C. S. Layered Na[Ni1/3Fe1/3Mn1/3]O2 cathodes for Na-ion battery application. Electrochem. Commun. 2012, 18, 66–69.
Komaba, S.; Yabuuchi, N.; Nakayama, T.; Ogata, A.; Ishikawa, T.; Nakai, I. Study on the reversible electrode reaction of Na1–xNi0.5Mn0.5O2 for a rechargeable sodium-ion battery. Inorg. Chem. 2012, 51, 6211–6220.
Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517.
Lei, Y. C.; Li, X.; Liu, L.; Ceder, G. Synthesis and stoichiometry of different layered sodium cobalt oxides. Chem. Mater. 2014, 26, 5288–5296.
Berthelot, R.; Carlier, D.; Delmas, C. Electrochemical investigation of the P2-NaxCoO2 phase diagram. Nat. Mater. 2011, 10, 74–80.
Kim, H.; Park, I.; Lee, S.; Kim, H.; Park, K.-Y.; Park, Y.-U.; Kim, H.; Kim, J.; Lim, H.-D.; Yoon, W.-S. et al. Understanding the electrochemical mechanism of the new iron-based mixed-phosphate Na4Fe3(PO4)2(P2O7) in a Na rechargeable battery. Chem. Mater. 2013, 25, 3614–3622.
Ha, K. H.; Woo, S. H.; Mok, D.; Choi, N. S.; Park, Y.; Oh, S. M.; Kim, Y.; Kim, J.; Lee, J.; Nazar, L. F. et al. Na4–αM2+α/2(P2O7)2 (2/3 ≤ α ≤ 7/8, M = Fe, Fe0.5Mn0.5, Mn): A promising sodium ion cathode for Na-ion batteries. Adv. Energy Mater. 2013, 3, 770–776.
Barpanda, P.; Ye, T.; Nishimura, S.-I.; Chung, S.-C.; Yamada, Y.; Okubo, M.; Zhou, H. S.; Yamada, A. Sodium iron pyrophosphate: A novel 3.0 V iron-based cathode for sodium-ion batteries. Electrochem. Commun. 2012, 24, 116–119.
Jian, Z. L.; Zhao, L.; Pan, H. L.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, L. Q. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem. Commun. 2012, 14, 86–89.
Moreau, P.; Guyomard, D.; Gaubicher, J.; Boucher, F. Structure and stability of sodium intercalated phases in olivine FePO4. Chem. Mater. 2010, 22, 4126–4128.
Ellis, B. L.; Makahnouk, W. R. M.; Rowan-Weetaluktuk, W. N.; Ryan, D. H.; Nazar, L. F. Crystal structure and electrochemical properties of A2MPO4F Fluorophosphates (A = Na, Li; M = Fe, Mn, Co, Ni). Chem. Mater. 2010, 22, 1059–1070.
Kim, S.-W.; Seo, D.-H.; Kim, H.; Park, K.-Y.; Kang, K. A comparative study on Na2MnPO4F and Li2MnPO4F for rechargeable battery cathodes. Phys. Chem. Chem. Phys. 2012, 14, 3299–3303.
Park, Y. U.; Seo, D. H.; Kim, H.; Kim, J.; Lee, S.; Kim, B.; Kang, K. A family of high-performance cathode materials for Na-ion batteries, Na3(VO1−xPO4)2F1+2x (0≤ x ≤ 1): Combined first-principles and experimental study. Adv. Funct. Mater. 2014, 24, 4603–4614.
Park, Y.-U.; Seo, D.-H.; Kwon, H.-S.; Kim, B.; Kim, J.; Kim, H.; Kim, I.; Yoo, H.-I.; Kang, K. A new high-energy cathode for a Na-ion battery with ultrahigh stability. J. Am. Chem. Soc. 2013, 135, 13870–13878.
Recham, N.; Chotard, J.-N.; Dupont, L.; Djellab, K.; Armand, M.; Tarascon, J.-M. Ionothermal synthesis of sodium-based fluorophosphate cathode materials. J. Electrochem. Soc. 2009, 156, A993–A999.
Barpanda, P.; Chotard, J.-N.; Recham, N.; Delacourt, C.; Ati, M.; Dupont, L.; Armand, M.; Tarascon, J.-M. Structural, transport, and electrochemical investigation of novel AMSO4F (A = Na, Li; M = Fe, Co, Ni, Mn) metal fluorosulphates prepared using low temperature synthesis routes. Inorg. Chem. 2010, 49, 7401–7413.
Dwibedi, D.; Ling, C. D.; Araujo, R. B.; Chakraborty, S.; Duraisamy, S.; Munichandraiah, N.; Ahuja, R.; Barpanda, P. Ionothermal synthesis of high-voltage Alluaudite Na2+2xFe2–x(SO4)3 sodium insertion compound: Structural, electronic, and magnetic insights. ACS Appl. Mater. Interfaces 2016, 8, 6982–6991.
Wei, S. H.; Mortemard de Boisse, B.; Oyama, G.; Nishimura, S. I.; Yamada, A. Synthesis and electrochemistry of Na2.5(Fe1−yMny)1.75(SO4)3 solid solutions for Na-ion batteries. ChemElectroChem 2016, 3, 209–213.
Ma, D.-L.; Wang, H.-G.; Li, Y.; Xu, D.; Yuan, S.; Huang, X.-L.; Zhang, X.-B.; Zhang, Y. In situ generated FeF3 in homogeneous iron matrix toward high-performance cathode material for sodium-ion batteries. Nano Energy 2014, 10, 295–304.
Nishijima, M.; Gocheva, I. D.; Okada, S.; Doi, T.; Yamaki, J.-I.; Nishida, T. Cathode properties of metal trifluorides in Li and Na secondary batteries. J. Power Sources 2009, 190, 558–562.
He, K.; Zhou, Y. N.; Gao, P.; Wang, L. P.; Pereira, N.; Amatucci, G. G.; Nam, K.-W.; Yang, X.-Q.; Zhu, Y. M.; Wang, F. et al. Sodiation via heterogeneous disproportionation in FeF2 electrodes for sodium-ion batteries. ACS Nano 2014, 8, 7251–7259.
Kim, S.-W.; Nam, K.-W.; Seo, D.-H.; Hong, J.; Kim, H.; Gwon, H.; Kang, K. Energy storage in composites of a redox couple host and a lithium ion host. Nano Today 2012, 7, 168–173.
Jung, S.-K.; Kim, H.; Cho, M. G.; Cho, S.-P.; Lee, B.; Kim, H.; Park, Y.-U.; Hong, J.; Park, K.-Y.; Yoon, G. et al. Lithium-free transition metal monoxides for positive electrodes in lithium-ion batteries. Nature Energy 2017, 2, 16208.
Dimov, N.; Kitajou, A.; Hori, H.; Kobayashi, E.; Okada, S. Electrochemical splitting of LiF: A new approach to lithium-ion battery materials. ECS Trans. 2014, 58, 87–99.
Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacin, M. R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583.
Shao, Y. J.; Yue, H. J.; Qiao, R. M.; Hu, J. Q.; Zhong, G. M.; Wu, S. Q.; McDonald, M. J.; Gong, Z. L.; Zhu, Z. Z.; Yang, W. L. et al. Synthesis and reaction mechanism of novel fluorinated carbon fiber as a high-voltage cathode material for rechargeable Na batteries. Chem. Mater. 2016, 28, 1026–1033.
Hudson, E.; Moler, E.; Zheng, Y.; Kellar, S.; Heimann, P.; Hussain, Z.; Shirley, D. Near-edge sodium and fluorine K-shell photoabsorption of alkali halides. Phys. Rev. B 1994, 49, 3701–3708.
Nakai, S.-I.; Ohashi, M.; Mitsuishi, T.; Maezawa, H.; Oizumi, H.; Fujikawa, T. F–K XANES studies of alkali fluorides. J. Phys. Soc. Jpn. 1986, 55, 2436–2442.
Krasnikov, S. A.; Vinogradov, A. S.; Preobrajenski, A. B.; Gridneva, L. K.; Molodtsov, S. L.; Laubschat, C.; Szargan, R. Electronic structure of FeF2 and FeF3 studied by X-ray absorption and fluorescence spectroscopy. Phys. Scr. 2005, 2005, 1074.
Kuzmin, A.; Parent, P. Focusing and superfocusing effects in X-ray absorption fine structure at the iron K edge in FeF3. J. Phys.: Condens. Matter 1994, 6, 4395.
Zhang, W.; Duchesne, P. N.; Gong, Z.-L.; Wu, S.-Q.; Ma, L.; Jiang, Z.; Zhang, S.; Zhang, P.; Mi, J.-X.; Yang, Y. In situ electrochemical XAFS studies on an iron fluoride highcapacity cathode material for rechargeable lithium batteries. J. Phys. Chem. C 2013, 117, 11498–11505.
Wang, F.; Kim, S.-W.; Seo, D.-H.; Kang, K.; Wang, L. P.; Su, D.; Vajo, J. J.; Wang, J.; Graetz, J. Ternary metal fluorides as high-energy cathodes with low cycling hysteresis. Nat. Commun. 2015, 6, 6668.
Li, L. S.; Jacobs, R.; Gao, P.; Gan, L. Y.; Wang, F.; Morgan, D.; Jin, S. Origins of large voltage hysteresis in high-energy-density metal fluoride lithium-ion battery conversion electrodes. J. Am. Chem. Soc. 2016, 138, 2838–2848.
Cosandey, F.; Al-Sharab, J. F.; Badway, F.; Amatucci, G. G.; Stadelmann, P. EELS spectroscopy of iron fluorides and FeFx/C nanocomposite electrodes used in Li-ion batteries. Microsc. Microanal. 2007, 13, 87–95.
Van Aken, P. A.; Liebscher, B.; Styrsa, V. J. Quantitative determination of iron oxidation states in minerals using Fe L2,3-edge electron energy-loss near-edge structure spectroscopy. Phys. Chem. Miner. 1998, 25, 323–327.
Augustyn, V.; Come, J.; Lowe, M. A.; Kim, J. W.; Taberna, P.-L.; Tolbert, S. H.; Abruña, H. D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522.
Acknowledgements
This work was supported by Samsung Research Funding Center of Samsung Electronics (No. SRFC-TA1403-03). This work was also supported by the World Premier Materials grant funded by the Korea government Ministry of Trade, Industry and Energy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hwang, I., Jung, SK., Jeong, ES. et al. NaF–FeF2 nanocomposite: New type of Na-ion battery cathode material. Nano Res. 10, 4388–4397 (2017). https://doi.org/10.1007/s12274-017-1538-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1538-y