Abstract
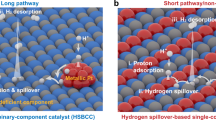
Hydrogen evolution by electrocatalysis is an attractive method of supplying clean energy. However, it is challenging to find cheap and efficient alternatives to rare and expensive platinum based catalysts. Pt provides the best hydrogen evolution performance, because it optimally balances the free energies of adsorption and desorption. Appropriate control of these quantities is essential for producing an efficient electrocatalyst. We demonstrate, based on first principles calculations, a stepwise designed Rh-Au-Si ternary catalyst, in which adsorption (the Volmer reaction) and desorption (the Heyrovsky reaction) take place on Rh and Si surfaces, respectively. The intermediate Au surface plays a vital role by promoting hydrogen diffusion from the Rh to the Si surface. Theoretical predictions have been explored extensively and verified by experimental observations. The optimized catalyst (Rh-Au-SiNW-2) has a composition of 2.2:28.5:69.3 (Rh:Au:Si mass ratio) and exhibits a Tafel slope of 24.0 mV·dec–1. Its electrocatalytic activity surpasses that of a commercial 40 wt.% Pt/C catalyst at overpotentials above 0.19 V by exhibiting a current density of greater than 108 mA·cm–2. At 0.3 V overpotential, the turnover frequency of Rh-Au-SiNW-2 is 10.8 times greater than that of 40 wt.% Pt/C. These properties may open new directions in the stepwise design of highly efficient catalysts for the hydrogen evolution reaction (HER).

Similar content being viewed by others
References
Armand, M.; Tarascon, J. M. Building better batteries. Nature 2008, 451, 652–657.
Potocnik, J. Renewable energy sources and the realities of setting an energy agenda. Science 2007, 315, 810–811.
Cook, T. R.; Dogutan, D. K.; Reece, S. Y.; Surendranath, Y.; Teets, T. S.; Nocera, D. G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502.
Morales-Guio, C. G.; Stern, L. A.; Hu, X. L. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569.
Yan, Y.; Xia, B. Y.; Xu, Z. C.; Wang, X. Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catal. 2014, 4, 1693–1705.
Zou, X. X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180.
Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S. Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem., Int. Ed. 2015, 54, 52–65.
Hinnemann, B.; Moses, P. G.; Bonde, J.; Jøgensen, K. P.; Nielsen, J. H.; Horch, S.; Chorkendorff, I.; Nøskov, J. K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309.
Greeley, J.; Jaramillo, T. F.; Bonde, J.; Chorkendorff, I.; Nø skov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913.
Huang, X.; Zeng, Z. Y.; Bao, S. Y.; Wang, M. F.; Qi, X. Y.; Fan, Z. X.; Zhang, H. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 2013, 4, 1444.
Esposito, D. V.; Hunt, S. T.; Stottlemyer, A. L.; Dobson, K. D.; McCandless, B. E.; Birkmire, R. W.; Chen, J. G. Low-cost hydrogen-evolution catalysts based on monolayer platinum on tungsten monocarbide substrates. Angew. Chem., Int. Ed. 2010, 49, 9859–9862.
Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K. C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260.
Li, Y. J.; Zhang, H. C.; Xu, T. H.; Lu, Z. Y.; Wu, X. C.; Wan, P. B.; Sun, X. M.; Jiang, L. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1737–1744.
Zhu, L. L.; Lin, H. P.; Li, Y. Y.; Liao, F.; Lifshitz, Y.; Sheng, M. Q.; Lee, S. T.; Shao, M. W. A rhodium/silicon Co-electrocatalyst design concept to surpass platinum hydrogen evolution activity at high overpotentials. Nat. Commun. 2016, 7, 12272.
Jaramillo, T. F.; Jørgensen, K. P.; Bonde, J.; Nielsen, J. H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102.
Khan, M.; Yousaf, A. B.; Chen, M. M.; Wei, C. S.; Wu, X. B.; Huang, N. D.; Qi, Z. M.; Li, L. B. Molybdenum sulfide/graphene-carbon nanotube nanocomposite material for electrocatalytic applications in hydrogen evolution reactions. Nano Res. 2016, 9, 837–848.
Xie, J. F.; Zhang, H.; Li, S.; Wang, R. X.; Sun, X.; Zhou, M.; Zhou, J. F.; Lou, X. W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813.
Ye, W.; Ren, C. H.; Liu, D. B.; Wang, C. M.; Zhang, N.; Yan, W. S.; Song, L.; Xiong, Y. J. Maneuvering charge polarization and transport in 2H-MoS2 for enhanced electrocatalytic hydrogen evolution reaction. Nano Res. 2016, 9, 2662–2671.
Fan, X. J.; Zhou, H. Q.; Guo, X. WC nanocrystals grown on vertically aligned carbon nanotubes: An efficient and stable electrocatalyst for hydrogen evolution reaction. ACS Nano 2015, 9, 5125–5134.
Yang, X. F.; Kimmel, Y. C.; Fu, J.; Koel, B. E.; Chen, J. G. G. Activation of tungsten carbide catalysts by use of an oxygen plasma pretreatment. ACS Catal. 2012, 2, 765–769.
Mao, S.; Wen, Z. H.; Ci, S. Q.; Guo, X. R.; Ostrikov, K.; Chen, J. H. Perpendicularly oriented MoSe2/graphene nanosheets as advanced electrocatalysts for hydrogen evolution. Small 2015, 11, 414–419.
Tang, H.; Dou, K. P.; Kaun, C. C.; Kuang, Q.; Yang, S. H. MoSe2 nanosheets and their graphene hybrids: Synthesis, characterization and hydrogen evolution reaction studies. J. Mater. Chem. A 2014, 2, 360–364.
Huang, Z. P.; Chen, Z. B.; Chen, Z. Z.; Lv, C. C.; Meng, H.; Zhang, C. Ni12P5 nanoparticles as an efficient catalyst for hydrogen generation via electrolysis and photoelectrolysis. ACS Nano 2014, 8, 8121–8129.
Popczun, E. J.; Mckone, J. R.; Read, C. G.; Biacchi, A. J.; Wiltrout, A. M.; Lewis, N. S.; Schaak, R. E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270.
Gong, M.; Wang, D. Y.; Chen, C. C.; Hwang, B. J.; Dai, H. J. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46.
Tian, J. Q.; Liu, Q.; Asiri, A. M.; Sun, X. P. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590.
Popczun, E. J.; Read, C. G.; Roske, C. W.; Lewis, N. S.; Schaak, R. E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem., Int. Ed. 2014, 53, 5427–5430.
Faber, M. S.; Dziedzic, R.; Lukowski, M. A.; Kaiser, N. S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061.
Lasia, A. Electrochemical Impedance Spectroscopy and its Applications; Springer: New York, 2014; pp 220–221.
Shao, M. W.; Cheng, L.; Zhang, X. H.; Ma, D. D. D.; Lee, S. T. Excellent photocatalysis of HF-treated silicon nanowires. J. Am. Chem. Soc. 2009, 131, 17738–17739.
Shao, M. W.; Shan, Y. Y.; Wong, N. B.; Lee, S. T. Silicon nanowire sensors for bioanalytical applications: Glucose and hydrogen peroxide detection. Adv. Funct. Mater. 2005, 15, 1478–1482.
Acknowledgements
The project was supported by National Natural Science Foundation of China (No. 91433111), Qing Lan Project, Collaborative Innovation Center of Suzhou Nano Science & Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jiang, B., Yang, L., Liao, F. et al. A stepwise-designed Rh-Au-Si nanocomposite that surpasses Pt/C hydrogen evolution activity at high overpotentials. Nano Res. 10, 1749–1755 (2017). https://doi.org/10.1007/s12274-017-1447-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1447-0