Abstract
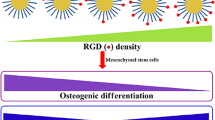
Chirality is one of the most distinctive biochemical signatures of life, and plays crucial roles in maintaining normal functions of living cells or organisms. Pioneering work from another group has demonstrated the dependency of cell differentiation on the chirality of nano-coated substrates, but the effect of the chiral surface of nanoparticles on stem cell fates has not been investigated. In this study, the influence of molecular chiral poly(acryloyl-L(D)-valine) (L(D)- PAV)-anchored gold nanoparticles (L(D)-PAV-AuNPs) on the differentiation of mesenchymal stem cells (MSCs) was investigated. Though osteogenic differentiation of MSCs was not affected by D-PAV-AuNPs, it was significantly promoted by L-PAV-AuNPs in terms of calcium deposition, alkaline phosphatase (ALP) activity, and expression of collagen type I and osteocalcin (OCN) at both mRNA and protein levels. L-PAV-AuNPs could activate the P38 mitogen-activated protein kinase (MAPK) pathway, and may exert mechanical stress on MSCs because of high amounts of internalization. These results provide new insights on surface chirality at the nanoscale as a direct regulator to guide the differentiation of MSCs, and the use of these nanomaterials for strategic regenerative medicine.

Similar content being viewed by others
References
Higuchi, A.; Ling, Q.-D.; Chang, Y.; Hsu, S.-T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 2013, 113, 3297–3328.
Langer, R.; Tirrell, D. A. Designing materials for biology and medicine. Nature 2004, 428, 487–492.
Khademhosseini, A.; Vacanti, J. P.; Langer, R. Progress in tissue engineering. Sci. Am. 2009, 300, 64–71.
Pittenger, M. F.; Mackay, A. M.; Beck, S. C.; Jaiswal, R. K.; Douglas, R.; Mosca, J. D.; Moorman, M. A.; Simonetti, D. W.; Craig, S.; Marshak, D. R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
Beachy, P. A.; Karhadkar, S. S.; Berman, D. M. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004, 432, 324–331.
Zhang, Z.-Y.; Teoh, S.-H.; Hui, J. H. P.; Fisk, N. M.; Choolani, M.; Chan, J. K. Y. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials 2012, 33, 2656–2672.
Zhang, Z. Y.; Teoh, S. H.; Chong, M. S. K.; Schantz, J. T.; Fisk, N. M.; Choolani, M. A.; Chan, J. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells 2009, 27, 126–137.
Seong, J. M.; Kim, B.-C.; Park, J.-H.; Kwon, I. K.; Mantalaris, A.; Hwang, Y.-S. Stem cells in bone tissue engineering. Biomed. Mater. 2010, 5, 062001.
Jiang, J. L.; Papoutsakis, E. T. Stem-cell niche based comparative analysis of chemical and nano-mechanical material properties impacting ex vivo expansion and differentiation of hematopoietic and mesenchymal stem cells. Adv. Healthc. Mater. 2013, 2, 25–42.
Benoit, D. S. W.; Schwartz, M. P.; Durney, A. R.; Anseth, K. S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008, 7, 816–823.
Curran, J. M.; Chen, R.; Hunt, J. A. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials 2006, 27, 4783–4793.
Kilian, K. A.; Bugarija, B.; Lahn, B. T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877.
Dingal, P. C. D. P.; Wells, R. G.; Discher, D. E. Simple insoluble cues specify stem cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 18104–18105.
Engler, A. J.; Sen, S.; Sweeney, H. L.; Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689.
McBeath, R.; Pirone, D. M.; Nelson, C. M.; Bhadriraju, K.; Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495.
Deng, J.; Sun, M. C.; Zhu, J. Y.; Gao, C. Y. Molecular interactions of different size AuNP–COOH nanoparticles with human fibrinogen. Nanoscale 2013, 5, 8130–8137.
Alkilany, A. M.; Lohse, S. E.; Murphy, C. J. The gold standard: Gold nanoparticle libraries to understand the nano–bio interface. Acc. Chem. Res. 2013, 46, 650–661.
Li, W.-J.; Tuli, R.; Huang, X. X.; Laquerriere, P.; Tuan, R. S. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 2005, 26, 5158–5166.
Xin, X. J.; Hussain, M.; Mao, J. J. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials 2007, 28, 316–325.
Ha, S.-W.; Weitzmann, M. N.; Beck, G. R., Jr. Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano 2014, 8, 5898–5910.
Li, J. J.; Kawazoe, N.; Chen, G. P. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials 2015, 54, 226–236.
Jiang, P. F.; Yu, D. H.; Zhang, W. J.; Mao, Z. W.; Gao, C. Y. Influence of bovine serum albumin coated poly(lactic-coglycolic acid) particles on differentiation of mesenchymal stem cells. RSC Adv. 2015, 5, 40924–40931.
Malcolm East, J. Membrane structural biology with biochemical and biophysical foundations. Mol. Membr. Biol. 2008, 25, 584.
Liu, G. F.; Zhang, D.; Feng, C. L. Control of three-dimensional cell adhesion by the chirality of nanofibers in hydrogels. Angew. Chem., Int. Ed. 2014, 53, 7789–7793.
Yao, X.; Hu, Y. W.; Cao, B.; Peng, R.; Ding, J. D. Effects of surface molecular chirality on adhesion and differentiation of stem cells. Biomaterials 2013, 34, 9001–9009.
Wang, X.; Gan, H.; Sun, T. L. Chiral design for polymeric biointerface: The influence of surface chirality on protein adsorption. Adv. Funct. Mater. 2011, 21, 3276–3281.
Yang, X.; Gan, L. F.; Han, L.; Li, D.; Wang, J.; Wang, E. K. Facile preparation of chiral penicillamine protected gold nanoclusters and their applications in cell imaging. Chem. Commun. 2013, 49, 2302–2304.
Li, Y.; Zhou, Y. L.; Wang, H. Y.; Perrett, S.; Zhao, Y. L.; Tang, Z. Y.; Nie, G. J. Chirality of glutathione surface coating affects the cytotoxicity of quantum dots. Angew. Chem., Int. Ed. 2011, 50, 5860–5864.
El-Sayed, M. A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264.
Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782.
Lind, U.; Greenidge, P.; Gustafsson, J.-Å.; Wright, A. P. H.; Carlstedt-Duke, J. Valine 571 functions as a regional organizer in programming the glucocorticoid receptor for differential binding of glucocorticoids and mineralocorticoids. J. Biol. Chem. 1999, 274, 18515–18523.
Li, M.; Tzagoloff, A. Assembly of the mitochondrial membrane system: Sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell 1979, 18, 47–53.
Gilbert, S. F.; Migeon, B. R. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell 1975, 5, 11–17.
Deng, J.; Li, Z.; Yao, M. Y.; Gao, C. Y. Influence of albumin configuration by the chiral polymer-grafted gold nanoparticles. Langmuir 2016, 32, 5608–5616.
Jiang, P. F.; Mao, Z. W.; Gao, C. Y. Combinational effect of matrix elasticity and alendronate density on differentiation of rat mesenchymal stem cells. Acta Biomater. 2015, 19, 76–84.
Deng, J.; Zheng, H. H.; Wu, S.; Zhang, P.; Gao, C. Y. Protein adsorption and cellular uptake of AuNPs capped with alkyl acids of different length. RSC Adv. 2015, 5, 22792–22801.
Gagner, J. E.; Lopez, M. D.; Dordick, J. S.; Siegel, R. W. Effect of gold nanoparticle morphology on adsorbed protein structure and function. Biomaterials 2011, 32, 7241–7252.
Röcker, C.; Pötzl, M.; Zhang, F.; Parak, W. J.; Nienhaus, G. U. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat. Nanotechnol. 2009, 4, 577–580.
Huang, R. X.; Carney, R. P.; Ikuma, K.; Stellacci, F.; Lau, B. L. T. Effects of surface compositional and structural heterogeneity on nanoparticle–protein interactions: Different protein configurations. ACS Nano 2014, 8, 5402–5412.
Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63.
Twentyman, P. R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285.
Yi, C. Q.; Liu, D. D.; Fong, C.-C.; Zhang, J. C.; Yang, M. S. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448.
Maxson, S.; Lopez, E. A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M. A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Trans. Med. 2012, 1, 142–149.
Stanford, C. M.; Jacobson, P. A.; Eanes, E. D.; Lembke, L. A.; Midura, R. J. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP). J. Biol. Chem. 1995, 270, 9420–9428.
Dalby, M. J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M. O.; Herzyk, P.; Wilkinson, C. D. W.; Oreffo, R. O. C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003.
Nikukar, H.; Reid, S.; Tsimbouri, P. M.; Riehle, M. O.; Curtis, A. S. G.; Dalby, M. J. Osteogenesis of mesenchymal stem cells by nanoscale mechanotransduction. ACS Nano 2013, 7, 2758–2767.
Abdallah, B. M.; Jensen, C. H.; Gutierrez, G.; Leslie, R. G. Q.; Jensen, T. G.; Kassem, M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J. Bone Miner. Res. 2004, 19, 841–852.
Pockwinse, S. M.; Wilming, L. G.; Conlon, D. M.; Stein, G. S.; Lian, J. B. Expression of cell growth and bone specific genes at single cell resolution during development of bone tissue-like organization in primary osteoblast cultures. J. Cell. Biochem. 1992, 49, 310–323.
Blomqvist, C.; Risteli, L.; Risteli, J.; Virkkunen, P.; Sarna, S.; Elomaa, I. Markers of type I collagen degradation and synthesis in the monitoring of treatment response in bone metastases from breast carcinoma. Br. J. Cancer 1996, 73, 1074–1079.
Nakamura, A.; Dohi, Y.; Akahane, M.; Ohgushi, H.; Nakajima, H.; Funaoka, H.; Takakura, Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng. Part C: Methods 2009, 15, 169–180.
Kratchmarova, I.; Blagoev, B.; Haack-Sorensen, M.; Kassem, M.; Mann, M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science 2005, 308, 1472–1477.
Zhang, W.; Liu, H. T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18.
Chang, L. F.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40.
Zeng, H. F.; Li, X. Y.; Xie, F.; Teng, L.; Chen, H. F. Dextran-coated fluorapatite nanorods doped with lanthanides in labelling and directing osteogenic differentiation of bone marrow mesenchymal stem cells. J. Mater. Chem. B 2014, 2, 3609–3617.
Choi, S. Y.; Song, M. S.; Ryu, P. D.; Lam, A. T. N.; Joo, S.-W.; Lee, S. Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int. J. Nanomedicine 2015, 10, 4383–4392.
Samberg, M. E.; Loboa, E. G.; Oldenburg, S. J.; Monteiro- Riviere, N. A. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine 2012, 7, 1197–1209.
Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
12274_2016_1239_MOESM1_ESM.pdf
Gold nanoparticles with surface-anchored chiral poly(acryloyl-L(D)-valine) induce differential response on mesenchymal stem cell osteogenesis
Rights and permissions
About this article
Cite this article
Deng, J., Zheng, H., Zheng, X. et al. Gold nanoparticles with surface-anchored chiral poly(acryloyl-L(D)-valine) induce differential response on mesenchymal stem cell osteogenesis. Nano Res. 9, 3683–3694 (2016). https://doi.org/10.1007/s12274-016-1239-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1239-y