Abstract
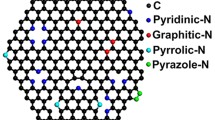
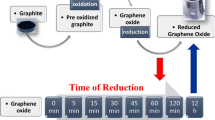
Chemical reduction of graphene oxide represents an important route towards large-scale production of graphene sheets for many applications. Thus far, gas-phase reactions have been demonstrated to efficiently reduce graphene oxide, but a molecular understanding of the reaction processes is largely lacking. Here, using molecular dynamics simulations, we compare the reduction of graphene oxide in different environments. We find that NH3 affords more efficient reduction of hydroxyl and epoxide groups than H2 and vacuum annealing partly due to lower energy barriers. Various reduction paths of oxygen groups in NH3 and H2 are quantitatively identified. Furthermore, we show that with the combination of vacancies and oxygen groups, pyridinic- or pyrrolic-like nitrogen can readily be incorporated into graphene. All of these nitrogen configurations lead to n-doping of the graphene. Our results are consistent with many previous experiments and provide insights towards doping engineering of graphene.

Similar content being viewed by others
References
Geim, A. K. Graphene: Status and prospects. Science 2009, 324, 1530–1534.
Geim, A. K.; Novoselov, K. S. The rise of graphene. Nat. Mater. 2007, 6, 183–191.
Park, S.; Ruoff, R. S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224.
Dreyer, D. R.; Park S.; Bielawski, C. W.; Ruoff, R. S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240.
Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir-Blodgett films. Nature Nanotech. 2008, 3, 538–542.
Dikin, D. A.; Stankovich, S.; Zimney, E. J.; Piner, R. D.; Dommett, G. H. B.; Evmenenko, G.; Nguyen, S. T.; Ruoff, R. S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460.
Stankovich, S.; Piner, R. D.; Chen, X.; Wu, N.; Nguyen, S. T.; Ruoff, R. S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158.
Gilje, S.; Han, S.; Wang, M.; Wang, K. L.; Kaner, R. B. A chemical route to graphene for device applications. Nano Lett. 2007, 7, 3394–3398.
Li, D.; Muller, M. B.; Gilje, S.; Kaner, R. B.; Wallace, G. G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105.
Wang, X.; Zhi, L.; Mullen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327.
Si, Y.; Samulski, E. T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682.
Suk, J. W.; Piner, R. D.; An, J.; Ruoff, R. S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564.
Wu, Z. -S.; Ren, W.; Gao, L.; Liu, B.; Jiang, C.; Cheng, H. -M. Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 2009, 47, 493–499.
Li, X.; Wang, H.; Robinson, J. T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous nitrogen doping and reduction of graphene oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944.
Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y. J.; Chhowalla, M.; Shenoy, V. B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587.
Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R. D.; Stankovich, S.; Jung, I.; Field, D. A.; Ventrice, C. A. Jr.; Ruoff, R. S. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and micro-Raman spectroscopy. Carbon 2009, 47, 145–152.
Jung, I.; Field, D. A.; Clark, N. J.; Zhu, Y.; Yang, D.; Piner, R. D.; Stankovich, S.; Dikin, D. A.; Geisler, H.; Ventrice, C. A., Jr. et al. Reduction kinetics of graphene oxide determined by electrical transport measurements and temperature pro- grammed desorption. J. Phys. Chem. C 2009, 113, 18480–18486.
Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472.
Stankovich, S.; Dikin, D. A.; Piner, R. D.; Kohlhaas, K. A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S. T.; Ruoff, R. S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565.
Shin, H. -J.; Kim, K. K.; Benayad, A.; Yoon, S. -M.; Park, H. K.; Jung, I. -S.; Jin, M. H.; Jeong, H. -K.; Kim, J. M.; Choi, J. -Y., et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992.
Jeong, H. -K.; Lee, Y. P.; Lahaye, R. J. W. E.; Park, M. -H.; An, K. H.; Kim, I. J.; Yang, C. -W.; Park, C. Y.; Ruoff, R. S.; Lee, Y. H. Evidence of graphitic AB stacking order of graphite oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366.
Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P. K.; Wang, H.; Guo, J.; Dai, H. N-doping of graphene through electro- thermal reactions with ammonia. Science 2009, 324, 768–771.
Guo, B.; Liu, Q.; Chen, E.; Zhu, H.; Fang, L.; Gong, J. R. Controllable N-doping of graphene. Nano Lett. 2010, 10, 4975–4980.
Jeong, H. M.; Lee, J. W.; Shin, W. H.; Choi, Y. J.; Shin, H. J.; Kang, J. K.; Choi, J. W. Nitrogen-doped graphene for high performance ultracapacitors and the importance of nitrogen- doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477.
Park, H. J.; Meyer, J.; Roth, S.; Skákalová, V. Growth and properties of few-layer graphene prepared by chemical vapor deposition. Carbon 2010, 48, 1088–1094.
Zhang, R. Q.; Chu, T. S.; Lee, C. S.; Lee, S. T. A theoretical study on the interactions of hydrogen species with various carbon and boron nitride phases. J. Phys. Chem. B 2000, 104, 6761–6766.
Reina, A.; Thiele, S.; Jia, X.; Bhaviripudi, S.; Dresselhaus, M.; Schaefer, J.; Kong, J. Growth of large-area single- and bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2009, 2, 509–516.
Dean J. A. Lange’s Handbook of Chemistry. 15th ed.; McGraw-Hill Book Company: New York, 1999.
Gao, W.; Alemany, L. B.; Ci, L.; Ajayan, P. M. New insights into structure and reduction of graphene oxide. Nat. Chem. 2009, 1, 403–408.
Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482.
Yan, J. -A.; Chou, M. Y. Oxidation functional groups on graphene: Structural and electronic properties. Phys. Rev. B 2010, 82, 125403.
Ghaderi, N.; Peressi, M. First-principle study of hydroxyl functional groups on pristine, defected graphene, and graphene epoxide. J. Phys. Chem. C 2010, 114, 21625–21630.
Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen- doped carbon nanotube arrays with high electro catalytic activity for oxygen reduction. Science 2009, 323, 760–764.
Qu, L.; Liu, Y.; Baek, J. -B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326.
Author information
Authors and Affiliations
Corresponding authors
Additional information
These authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xu, S., Dong, J., Pan, L. et al. A molecular understanding of the gas-phase reduction and doping of graphene oxide. Nano Res. 5, 361–368 (2012). https://doi.org/10.1007/s12274-012-0216-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-012-0216-3