Abstract
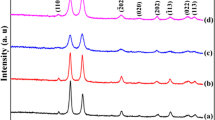
A series of unique nanowire superstructures, Cu2O nanowire polyhedra, have been synthesized through a cost-effective hydrothermal route. Three types of nanowire polyhedra, namely octahedra, concave octahedra, and hexapods, were formed in high morphological yields (90%) by reducing cupric acetate with o-anisidine or o-phenetidine in the presence of carboxylic acids. The architectures of these Cu2O nanowire polyhedra were examined by electron microscopy, which revealed ordered, highly aligned Cu2O nanowires within the polyhedral outlines. The growth of the Cu2O nanowire polyhedra is controlled by the orientation and growth rates of the nanowire branches which are adjusted by addition of carboxylic acids. Compared to the Cu2O samples reported in the recent literature, the Cu2O nanowire octahedra exhibit notably enhanced photocatalytic activities for dye degradation in the presence of H2O2 under visible light, probably due to the high-density charge carriers photoexcited from the branched nanowires with their special structures. Additionally, the discussion in the recent literature of the photocatalytic activity of Cu2O in the absence of H2O2 for direct photodegradation of dyes seems questionable.

Similar content being viewed by others
References
Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly monodisperse Cu2O and CuO nanospheres: Preparation and applications for sensitive gas sensors. Chem. Mater. 2006, 18, 867–871.
Zhang, H.; Zhu, Q.; Zhang, Y.; Wang, Y.; Zhao, L.; Yu, B. One-pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv. Funct. Mater. 2007, 17, 2766–2771.
Yao, K. X.; Yin, X. M.; Wang, T. H.; Zeng, H. C. Synthesis, self-assembly, disassembly, and reassembly of two types of Cu2O nanocrystals unifaceted with {001} or {110} planes. J. Am. Chem. Soc. 2010, 132, 6131–6144.
White, B.; Yin, M.; Hall, A.; Le, D.; Stolbov, S.; Rahman, T.; Turro, N.; O’Brien, S. Complete CO oxidation over Cu2O nanoparticles supported on silica gel. Nano Lett. 2006, 6, 2095–2098.
Niu, F.; Jiang, Y.; Song W. In situ loading of Cu2O nanoparticles on a hydroxyl group rich TiO2 precursor as an excellent catalyst for the Ullmann reaction. Nano Res. 2010, 3, 757–763.
Leng, M.; Liu, M.; Zhang, Y.; Wang, Z.; Yu, C.; Yang, X.; Zhang, H.; Wang, C. Polyhedral 50-facet Cu2O microcrystals partially enclosed by {311} high-index planes: Synthesis and enhanced catalytic CO oxidation activity. J. Am. Chem. Soc. 2010, 132, 17084–17087.
de Jongh, P. E.; Vanmaelkelbergh, D.; Kelly, J. J. Cu2O: A catalyst for the photochemical decomposition of water? Chem. Commun. 1999, 1069–1070.
Xu, H.; Wang, W.; Zhu, W. Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J. Phys. Chem. B 2006, 110, 13829–13834.
Kuo, C. -H.; Huang, M. H. Facile synthesis of Cu2O nanocrystals with systematic shape evolution from cubic to octahedral structures. J. Phys. Chem. C 2008, 112, 18355–18360.
Kuo, C. -H.; Chen, C. -H.; Huang, M. H. Seed-mediated synthesis of monodispersed Cu2O nanocubes with five different size ranges from 40 to 420 nm. Adv. Funct. Mater. 2007, 17, 3773–3780.
Gao, J.; Li, Q.; Zhao, H.; Li, L.; Liu, C.; Gong, Q.; Qi, L. One-pot synthesis of uniform Cu2O and CuS hollow spheres and their optical limiting properties. Chem. Mater. 2008, 20, 6263–6269.
Yuhas, B. D.; Yang, P. Nanowire-based all-oxide solar cells. J. Am. Chem. Soc. 2009, 131, 3756–3761.
Deki, S.; Akamatsu, K.; Yano, T.; Mizuhata, M.; Kajinami, A. Preparation and characterization of copper(I) oxide nanoparticles dispersed in a polymer matrix. J. Mater. Chem. 1998, 8, 1865–1868.
Rockenberger, J.; Scher, E. C.; Alivisatos, A. P. A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal oxides. J. Am. Chem. Soc. 1999, 121, 11595–11560.
Borgohain, K.; Murase, N.; Mahamuni, S. Synthesis and properties of Cu2O quantum particles. J. Appl. Phys. 2002, 92, 1292–1297.
Yin, M.; Wu, C. -K.; Lou, Y.; Burda, C.; Koberstein, J. T.; Zhu, Y.; O’Brien, S. Copper oxide nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511.
Gou, L.; Murphy, C. J. Controlling the size of Cu2O nanocubes from 200 to 25 nm J. Mater. Chem. 2004, 14, 735–738.
Ng, C. H. B.; Fan, W. Y. Shape evolution of Cu2O nanostructures via kinetic and thermodynamic controlled growth. J. Phys. Chem. B 2006, 110, 20801–20807.
Xu, H.; Wang, W. Template synthesis of multishelled Cu2O hollow spheres with a single-crystalline shell wall. Angew. Chem. Int. Ed. 2007, 46, 1489–1492.
Chang, Y.; Teo, J. J.; Zeng, H. C. Formation of colloidal CuO nanocrystallites and their spherical aggregation and reductive transformation to hollow Cu2O nanospheres. Langmuir 2005, 21, 1074–1079.
Teo, J. J.; Chang, Y.; Zeng, H. C. Fabrications of hollow nanocubes of Cu2O and Cu via reductive self-assembly of CuO nanocrystals. Langmuir 2006, 22, 7369–7377.
Lu, C.; Qi, L.; Yang, J.; Wang, X.; Zhang, D.; Xie, J.; Ma, J. One-pot synthesis of octahedral Cu2O nanocages via a catalytic solution route. Adv. Mater. 2005, 17, 2562–2567.
Kuo, C. -H.; Huang, M. H. Fabrication of truncated rhombic dodecahedral Cu2O nanocages and nanoframes by particle aggregation and acidic etching. J. Am. Chem. Soc. 2008, 130, 12815–12820.
Wang, W.; Wang, G.; Wang, X.; Zhan, Y.; Liu, Y.; Zheng, C. Synthesis and characterization of Cu2O nanowires by a novel reduction route. Adv. Mater. 2002, 14, 67–69.
Xiong, Y.; Li, Z.; Zhang, R.; Xie, Y.; Yang, J.; Wu, C. From complex chains to 1D metal oxides, a novel strategy to Cu2O nanowires. J. Phys. Chem. B 2003, 107, 3697–3702.
Tan, Y.; Xue, X.; Peng, Q.; Zhao, H.; Wang, T.; Li, Y. Controllable fabrication and electrical performance of single crystalline Cu2O nanowires with high aspect ratios. Nano Lett. 2007, 7, 3723–3728.
Ge, J. -P.; Wang, J.; Zhang, H. -X.; Wang, X.; Peng, Q.; Li, Y. D. Orthogonal PbS nanowire arrays and networks and their Raman scattering behavior. Chem. Eur. J. 2005, 11, 1889–1894.
Zhou, J.; Ding, Y.; Deng, S. Z.; Gong, L.; Xu, N. S.; Wang, Z. L. Three-dimensional tungsten oxide nanowire networks. Adv. Mater. 2005, 17, 2107–2110.
Zhu, J.; Peng, H.; Chan, C. K.; Jarausch, K.; Zhang, X. F.; Cui, Y. Hyperbranched lead selenide nanowire networks. Nano Lett. 2007, 7, 1095–1099.
Bierman, M. J.; Lau, Y. K. A.; Jin, S. Hyperbranched PbS and PbSe nanowires and the effect of hydrogen gas on their synthesis. Nano Lett. 2007, 7, 2907–2912.
He, J. H.; Ho, C. H.; Wang, C. W.; Ding, Y.; Chen, L. J.; Wang, Z. L. Growth of crossed ZnO nanorod networks induced by polar substrate surface. Cryst. Growth Des. 2009, 9, 17–19.
Gu, Z.; Liu, F.; Howe, J. Y.; Paranthaman, M. P.; Pan, Z. Three-dimensional germanium oxide nanowire networks. Cryst. Growth Des. 2009, 9, 35–39.
Wu, Y.; Livneh, T.; Zhang, Y. X.; Cheng, G.; Wang, J.; Tang, J.; Moskovits, M.; Stucky, G. D. Templated synthesis of highly ordered mesostructured nanowires and nanowire arrays. Nano Lett. 2004, 4, 2337–2342.
Wang, D.; Qian, F.; Yang, C.; Zhong, Z.; Lieber, C. M. Rational growth of branched and hyperbranched nanowire structures. Nano Lett. 2004, 4, 871–874.
Sun, S.; Zhou, F.; Wang, L.; Song, X.; Yang, Z. Templatefree synthesis of well-defined truncated edge polyhedral Cu2O architectures. Cryst. Growth Des. 2010, 10, 541–547.
Chang, Y.; Zeng, H. C. Manipulative synthesis of multipod frameworks for self-organization and self-amplification of Cu2O microcrystals. Cryst. Growth Des. 2004, 4, 273–278.
Hull, K. L.; Grebinski, J. W.; Kosel, T. H.; Kuno, M. Induced branching in confined PbSe nanowires. Chem. Mater. 2005, 17, 4416–4425.
Zhang, Z.; Wan, M.; Wei, Y. Highly crystalline polyaniline nanostructures doped with dicarboxylic acids. Adv. Funct. Mater. 2006, 16, 1100–1104.
Pan, L.; Pu, L.; Shi, Y.; Sun, T.; Zhang, R.; Zheng, Y. Hydrothermal synthesis of polyaniline mesostructures. Adv. Funct. Mater. 2006, 16, 1279–1288.
Tatsuma, T.; Tachibana, S. -i.; Miwa, T.; Tryk, D. A.; Fujishima, A. Remote bleaching of methylene blue by UV-irradiated TiO2 in the gas phase. J. Phys. Chem. B 1999, 103, 8033–8035.
Yan, S. C.; Li, Z. S.; Zou, Z. G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901.
Schöppel, H. R.; Gerischer, H. The cathodic reduction of Cu-I-oxide electrodes as an example of the mechanism of reduction of a semiconductor crystal. Ber. Bunsenges Phys. Chem. 1971, 75, 1237–1239.
Boyer, C.; Gamburzev, S.; Velev, O.; Srinivasan, S.; Appleby, A. J. Measurements of proton conductivity in the active layer of PEM fuel cell gas diffusion electrodes. Electrochim. Acta 1998, 43, 3703–3709.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shi, J., Li, J., Huang, X. et al. Synthesis and enhanced photocatalytic activity of regularly shaped Cu2O nanowire polyhedra. Nano Res. 4, 448–459 (2011). https://doi.org/10.1007/s12274-011-0101-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-011-0101-5