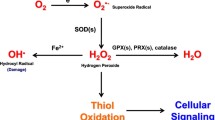
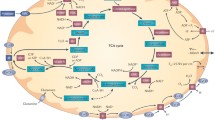
Abstract
In normal cells, the cellular reactive oxygen species (ROS) level is proportional to the activity of mitochondrial electron transport and tightly controlled by endogenous antioxidant system. However, energy metabolism and ROS homeostasis in cancer cells are much different from those in normal cells. For example, a majority of cellular glucose is metabolized through aerobic glycolysis (“Warburg effect”) and the pentose phosphate pathway. Cancer cells harbor functional mitochondria, but many mutations in nuclear DNA-encoded mitochondrial genes and mitochondrial genome result in the mitochondrial metabolic reprogramming. The other characteristic of cancer cells is to maintain much higher ROS level than normal cells. Ironically, cancer cells overexpress the ROS-producing NADPH oxidase and the ROS-eliminating antioxidant enzymes, both of which enzyme systems share NADPH as a reducing power source. In this article, we review the complex connection between ROS and energy metabolisms in cancer cells.




Similar content being viewed by others
References
Anastasiou, D., G. Poulogiannis, J.M. Asara, M.B. Boxer, J.K. Jiang, M. Shen, G. Bellinger, et al. 2011. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334(6060): 1278–1283.
Arnold, R.S., J. Shi, E. Murad, A.M. Whalen, C.Q. Sun, R. Polavarapu, S. Parthasarathy, J.A. Petros, and J.D. Lambeth. 2001. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proceedings of the National Academy of Sciences of the United States of America 98(10): 5550–5555.
Baliga, M.S., H. Wang, P. Zhuo, J.L. Schwartz, and A.M. Diamond. 2007. Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biological Trace Element Research 115(3): 227–242.
Bedard, K., and K.H. Krause. 2007. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiological Reviews 87(1): 245–313.
Bensaad, K., A. Tsuruta, M.A. Selak, M.N. Vidal, K. Nakano, R. Bartrons, E. Gottlieb, and K.H. Vousden. 2006. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126(1): 107–120.
Biteau, B., J. Labarre, and M.B. Toledano. 2003. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425(6961): 980–984.
Bonner, M.Y., and J.L. Arbiser. 2012. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cellular and Molecular Life Sciences 69(14): 2435–2442.
Chae, H.Z., H.J. Kim, S.W. Kang, and S.G. Rhee. 1999. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Research and Clinical Practice 45(2–3): 101–112.
Chang, T.S., C.S. Cho, S. Park, S. Yu, S.W. Kang, and S.G. Rhee. 2004. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. Journal of Biological Chemistry 279(40): 41975–41984.
Choi, H., S. Kim, P. Mukhopadhyay, S. Cho, J. Woo, G. Storz, and S.E. Ryu. 2001. Structural basis of the redox switch in the OxyR transcription factor. Cell 105(1): 103–113.
Choi, M.H., I.K. Lee, G.W. Kim, B.U. Kim, Y.H. Han, D.Y. Yu, H.S. Park, et al. 2005. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 435(7040): 347–353.
Christofk, H.R., M.G. Vander Heiden, N. Wu, J.M. Asara, and L.C. Cantley. 2008. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452(7184): 181–186.
Cosentino, C., D. Grieco, and V. Costanzo. 2011. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO Journal 30(3): 546–555.
Crosas-Molist, E., E. Bertran, P. Sancho, J. Lopez-Luque, J. Fernando, A. Sanchez, M. Fernandez, E. Navarro, and I. Fabregat. 2014. The NADPH oxidase NOX4 inhibits hepatocyte proliferation and liver cancer progression. Free Radical Biology and Medicine 69: 338–347.
Cullen, J.J., F.A. Mitros, and L.W. Oberley. 2003. Expression of antioxidant enzymes in diseases of the human pancreas: Another link between chronic pancreatitis and pancreatic cancer. Pancreas 26(1): 23–27.
D’Alessandro, A., I. Amelio, C.R. Berkers, A. Antonov, K.H. Vousden, G. Melino, and L. Zolla. 2014. Metabolic effect of TAp63alpha: Enhanced glycolysis and pentose phosphate pathway, resulting in increased antioxidant defense. Oncotarget 5(17): 7722–7733.
Du, W., P. Jiang, A. Mancuso, A. Stonestrom, M.D. Brewer, A.J. Minn, T.W. Mak, M. Wu, and X. Yang. 2013. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nature Cell Biology 15(8): 991–1000.
Eaton, P., N. Wright, D.J. Hearse, and M.J. Shattock. 2002. Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. Journal of Molecular and Cellular Cardiology 34(11): 1549–1560.
Ergen, H.A., U. Gormus, F. Narter, U. Zeybek, S. Bulgurcuoglu, and T. Isbir. 2007. Investigation of NAD(P)H:quinone oxidoreductase 1 (NQO1) C609T polymorphism in prostate cancer. Anticancer Research 27(6B): 4107–4110.
Fagerholm, R., B. Hofstetter, J. Tommiska, K. Aaltonen, R. Vrtel, K. Syrjakoski, A. Kallioniemi, et al. 2008. NAD(P)H:quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nature Genetics 40(7): 844–853.
Fan, J., J. Ye, J.J. Kamphorst, T. Shlomi, C.B. Thompson, and J.D. Rabinowitz. 2014. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510(7504): 298–302.
Favaro, E., K. Bensaad, M.G. Chong, D.A. Tennant, D.J. Ferguson, C. Snell, G. Steers, et al. 2012. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metabolism 16(6): 751–764.
Gladyshev, V.N., V.M. Factor, F. Housseau, and D.L. Hatfield. 1998. Contrasting patterns of regulation of the antioxidant selenoproteins, thioredoxin reductase, and glutathione peroxidase, in cancer cells. Biochemical and Biophysical Research Communications 251(2): 488–493.
Guo, Z., S. Kozlov, M.F. Lavin, M.D. Person, and T.T. Paull. 2010. ATM activation by oxidative stress. Science 330(6003): 517–521.
Guptasarma, P., D. Balasubramanian, S. Matsugo, and I. Saito. 1992. Hydroxyl radical mediated damage to proteins, with special reference to the crystallins. Biochemistry 31(17): 4296–4303.
Hwang, N.R., S.H. Yim, Y.M. Kim, J. Jeong, E.J. Song, Y. Lee, J.H. Lee, S. Choi, and K.J. Lee. 2009. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. The Biochemical Journal 423(2): 253–264.
Ishii, T., O. Sunami, H. Nakajima, H. Nishio, T. Takeuchi, and F. Hata. 1999. Critical role of sulfenic acid formation of thiols in the inactivation of glyceraldehyde-3-phosphate dehydrogenase by nitric oxide. Biochemical Pharmacology 58(1): 133–143.
Jeon, S.M., N.S. Chandel, and N. Hay. 2012. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485(7400): 661–665.
Jeon, S.M., S.J. Lee, T.K. Kwon, K.J. Kim, and Y.S. Bae. 2010. NADPH oxidase is involved in protein kinase CKII down-regulation-mediated senescence through elevation of the level of reactive oxygen species in human colon cancer cells. FEBS Letters 584(14): 3137–3142.
Jeong, W., S.J. Park, T.S. Chang, D.Y. Lee, and S.G. Rhee. 2006. Molecular mechanism of the reduction of cysteine sulfinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. Journal of Biological Chemistry 281(20): 14400–14407.
Jiang, P., W. Du, X. Wang, A. Mancuso, X. Gao, M. Wu, and X. Yang. 2011. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nature Cell Biology 13(3): 310–316.
Jiang, P., W. Du, and X. Yang. 2013. A critical role of glucose-6-phosphate dehydrogenase in TAp73-mediated cell proliferation. Cell Cycle 12(24): 3720–3726.
Kang, D.H., D.J. Lee, K.W. Lee, Y.S. Park, J.Y. Lee, S.H. Lee, Y.J. Koh, et al. 2011. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Molecular Cell 44(4): 545–558.
Kang, S.W., S.G. Rhee, T.S. Chang, W. Jeong, and M.H. Choi. 2005. 2-Cys peroxiredoxin function in intracellular signal transduction: Therapeutic implications. Trends in Molecular Medicine 11(12): 571–578.
Kato, K., T. Ogura, A. Kishimoto, Y. Minegishi, N. Nakajima, M. Miyazaki, and H. Esumi. 2002. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 21(39): 6082–6090.
Laderoute, K.R., K. Amin, J.M. Calaoagan, M. Knapp, T. Le, J. Orduna, M. Foretz, and B. Viollet. 2006. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Molecular and Cellular Biology 26(14): 5336–5347.
Liu, C.X., Q.Q. Yin, H.C. Zhou, Y.L. Wu, J.X. Pu, L. Xia, W. Liu, et al. 2012. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nature Chemical Biology 8(5): 486–493.
Locasale, J.W. 2013. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nature Reviews Cancer 13(8): 572–583. doi:10.1038/nrc3557.
Lubos, E., J. Loscalzo, and D.E. Handy. 2011. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants & Redox Signaling 15(7): 1957–1997.
Maddocks, O.D., C.R. Berkers, S.M. Mason, L. Zheng, K. Blyth, E. Gottlieb, and K.H. Vousden. 2013. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493(7433): 542–546.
Morigasaki, S., K. Shimada, A. Ikner, M. Yanagida, and K. Shiozaki. 2008. Glycolytic enzyme GAPDH promotes peroxide stress signaling through multistep phosphorelay to a MAPK cascade. Molecular Cell 30(1): 108–113.
Muchowicz, A., M. Firczuk, J. Chlebowska, D. Nowis, J. Stachura, J. Barankiewicz, A. Trzeciecka, et al. 2014. Adenanthin targets proteins involved in the regulation of disulphide bonds. Biochemical Pharmacology 89(2): 210–216.
Newmeyer, D.D., and S. Ferguson-Miller. 2003. Mitochondria: Releasing power for life and unleashing the machineries of death. Cell 112(4): 481–490.
Park, H.S., J.N. Chun, H.Y. Jung, C. Choi, and Y.S. Bae. 2006. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovascular Research 72(3): 447–455.
Parzefall, W., C. Freiler, O. Lorenz, H. Koudelka, T. Riegler, M. Nejabat, E. Kainzbauer, B. Grasl-Kraupp, and R. Schulte-Hermann. 2014. Superoxide deficiency attenuates promotion of hepatocarcinogenesis by cytotoxicity in NADPH oxidase knockout mice. Archives of Toxicology, 1–11.
Ralser, M., M.M. Wamelink, A. Kowald, B. Gerisch, G. Heeren, E.A. Struys, E. Klipp, et al. 2007. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. Journal of Biology 6(4): 10.
Rodacka, A., J. Strumillo, E. Serafin, and M. Puchala. 2014. Effect of resveratrol and tiron on the inactivation of glyceraldehyde-3-phosphate dehydrogenase induced by superoxide anion radical. Current Medicinal Chemistry 21(8): 1061–1069.
Salmeen, A., J.N. Andersen, M.P. Myers, T.C. Meng, J.A. Hinks, N.K. Tonks, and D. Barford. 2003. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423(6941): 769–773.
Shacter, E. 2000. Quantification and significance of protein oxidation in biological samples. Drug Metabolism Reviews 32(3–4): 307–326.
Soethoudt, M., A.V. Peskin, N. Dickerhof, L.N. Paton, P.E. Pace, and C.C. Winterbourn. 2014. Interaction of adenanthin with glutathione and thiol enzymes: Selectivity for thioredoxin reductase and inhibition of peroxiredoxin recycling. Free Radical Biology and Medicine 77: 331–339.
Souza, J.M., and R. Radi. 1998. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Archives of Biochemistry and Biophysics 360(2): 187–194.
Suh, Y.A., R.S. Arnold, B. Lassegue, J. Shi, X. Xu, D. Sorescu, A.B. Chung, K.K. Griendling, and J.D. Lambeth. 1999. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401(6748): 79–82.
Sullivan, L.B., E. Martinez-Garcia, H. Nguyen, A.R. Mullen, E. Dufour, S. Sudarshan, J.D. Licht, R.J. Deberardinis, and N.S. Chandel. 2013. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Molecular Cell 51(2): 236–248.
Sundaresan, M., Z.X. Yu, V.J. Ferrans, K. Irani, and T. Finkel. 1995. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270(5234): 296–299.
Tonks, N.K. 2005. Redox redux: Revisiting PTPs and the control of cell signaling. Cell 121(5): 667–670.
Tonks, N.K. 2006. Protein tyrosine phosphatases: From genes, to function, to disease. Nature Reviews Molecular Cell Biology 7(11): 833–846.
Trachootham, D., J. Alexandre, and P. Huang. 2009. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews Drug Discovery 8(7): 579–591.
Treton, X., E. Pedruzzi, C. Guichard, Y. Ladeiro, S. Sedghi, M. Vallee, N. Fernandez, et al. 2014. Combined NADPH oxidase 1 and interleukin 10 deficiency induces chronic endoplasmic reticulum stress and causes ulcerative colitis-like disease in mice. PLoS One 9(7): e101669.
van Montfort, R.L., M. Congreve, D. Tisi, R. Carr, and H. Jhoti. 2003. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature 423(6941): 773–777.
Vander Heiden, M.G., L.C. Cantley, and C.B. Thompson. 2009. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324(5930): 1029–1033.
Vaughn, A.E., and M. Deshmukh. 2008. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nature Cell Biology 10(12): 1477–1483.
Wallace, D.C. 2012. Mitochondria and cancer. Nature Reviews Cancer 12(10): 685–698.
Wang, W., H. Fang, L. Groom, A. Cheng, W. Zhang, J. Liu, X. Wang, et al. 2008. Superoxide flashes in single mitochondria. Cell 134(2): 279–290.
Woo, H.A., H.Z. Chae, S.C. Hwang, K.S. Yang, S.W. Kang, K. Kim, and S.G. Rhee. 2003. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science 300(5619): 653–656.
Woo, H.A., S.H. Yim, D.H. Shin, D. Kang, D.Y. Yu, and S.G. Rhee. 2010. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 140(4): 517–528.
Wood, Z.A., L.B. Poole, and P.A. Karplus. 2003. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300(5619): 650–653.
Yang, C.S., D.M. Shin, K.H. Kim, Z.W. Lee, C.H. Lee, S.G. Park, Y.S. Bae, and E.K. Jo. 2009. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. Journal of Immunology 182(6): 3696–3705.
Yang, K.S., S.W. Kang, H.A. Woo, S.C. Hwang, H.Z. Chae, K. Kim, and S.G. Rhee. 2002. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. Journal of Biological Chemistry 277(41): 38029–38036.
Yoo, S.K., T.W. Starnes, Q. Deng, and A. Huttenlocher. 2011. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480(7375): 109–112.
Zhang, Q., M. Zheng, X.L. Qi, F. Liu, Z.J. Mao, and D.H. Zhang. 2014. Effect of NQO1 C609T polymorphism on prostate cancer risk: a meta-analysis. Onco Targets and Therapy 7: 907–914.
Zmijewski, J.W., S. Banerjee, H. Bae, A. Friggeri, E.R. Lazarowski, and E. Abraham. 2010. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. Journal of Biological Chemistry 285(43): 33154–33164.
Acknowledgments
This study was supported by a Grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1420280), the National Research Foundation Grants (2014R1A2A1A01006934 & 2012M3A9C5048709), and the Research Center for Cellular Homeostasis (2012R1A5A1048236) funded by the Korean government (MSIP).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, S.W., Lee, S. & Lee, E.K. ROS and energy metabolism in cancer cells: alliance for fast growth. Arch. Pharm. Res. 38, 338–345 (2015). https://doi.org/10.1007/s12272-015-0550-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0550-6