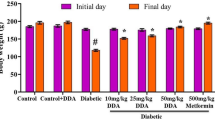
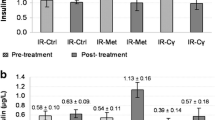
Abstract
Our aims were to investigate the hypoglycemic effects and mechanisms of action of Ganoderma lucidum polysaccharides (GLPs) administered for 7 days in type 2 diabetic mice. The mice were randomly divided into four groups (8 mice/group): normal control group, diabetic control group, low-dose GLP-treated diabetic group (50 mg/kg/d), and high-dose GLP-treated diabetic group (100 mg/kg/d). Diabetes was induced by streptozotocin injection and high-fat dietary feeding. At the end of the study, fasting serum glucose, insulin, body weight (BW) and epididymal white adipose tissue weight were measured. The hepatic mRNA levels of glycogen phosphorylase (GP), fructose-1,6-bisphosphatase (FBPase), phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) genes were determined by real-time polymerase chain reaction. Both doses of GLPs significantly decreased fasting serum glucose, insulin and epididymal fat/BW ratio compared with the diabetic control group (p < 0.05). The hepatic mRNA levels of GP, FBPase, PEPCK and G6Pase were significantly lower in both GLP-treated groups compared with the diabetic control group. Taken together, GLPs significantly decrease fasting serum glucose levels in type 2 diabetic mice in a dose-dependent manner. The decreases in fasting serum glucose levels may be associated with decreased mRNA expression levels of several key enzymes involved in gluconeogenesis and/or glycogenolysis.
Similar content being viewed by others
References
Agius, L., New hepatic targets for glycaemic control in diabetes. Best Pract. Res. Clin. Endocrinol. Metab., 21, 587–605 (2007).
Aoki, K., Kikuchi, T., Mukasa, K., Ito, S., Nakajima, A., Satoh, S., Okamura, A., and Sekihara, H., Dehydroepiandrosterone suppresses elevated hepatic glucose-6-phosphatase mRNA level in C57BL/KsJ-db/db mice: comparison with troglitazone. Endocr. J., 47, 799–804 (2000).
Argaud, D., Zhang, Q., Pan, W. S., Maitra, S., Pilkis, S. J., Lange, A. J., Regulation of rat liver glucose-6-phosphatase gene expression in different nutritional and hormonal states — Gene structure and 5′-flanking sequence. Diabetes, 45, 1563–1571 (1996).
Brink, M., Price, S. R., Chrast, J., Bailey, J. L., Anwar, A., Mitch, W. E., and Delafontaine, P., Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology, 142, 1489–1496 (2001).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F., Colorimetric method for determination of sugars and related substances. Anal. Chem., 28, 350–356 (1956).
Hanson, R. W. and Reshef, L., Regulation of phosphoenolpyruvate carboxykinase (GTP) gene. Annu. Rev. Biochem., 66, 581–611 (1997).
Hikino, H., Konno, C., Mirin, Y., and Hayashi, T., Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Med., 51, 339–340 (1985).
Hikino, H., Ishiyama, M., Suzuki, Y., and Konno, C., Mechanisms of hypoglycemic activity of ganoderan B: a glycan of Ganoderma lucidum fruit bodies. Planta Med., 55, 423–428 (1989).
Hotta, K., Kuwajima, M., Ono, A., Nakajima, H., Shingu, R., Miyagawa, J., Namba, M., Hanafusa, T., Noguchi, T., Kono, N., and Matsuzawa, Y., Disordered expression of hepatic glycolytic and gluconeogenic enzymes in Otsuka Long-Evans Tokushima fatty rats with spontanteous longterm hyperglycemia. Biochim. Biophys. Acta, 1289, 145–149 (1996).
Huang, X. L., Wu, H. Q., Huang, F., and Lin, X. S., Analysis of polysaccharide from broken cellular wall and unbroken spore of Ganoderma lucidum. Chinese Traditional and Herbal Drugs, 37, 813–816 (2006).
Kennaway, D. J., Owens, J. A., Voultsios, A., Boden, M. J., and Varcoe, T. J., Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol., 293, 1528–1537 (2007).
Kiho, T., Hui, J., Yamane, A., and Ukai, S., Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol. Pharm. Bull., 16, 1291–1293 (1993).
Kiho, T., Tsujimura, Y., Sakushima, M., Usui, S., and Ukai, S., Polysaccharides in fungi. XXXIII. Hypoglycemic activity of an acidic polysaccharide (AC) from Tremella fuciformis. Yakugaku Zasshi, 114, 308–315 (1994a).
Kiho T, Sobue S, Ukai S. Structural features and hypoglycemic activities of two polysaccharides from a hot-water extract of Agrocybe cylindracea. Carbohydr. Res., 251, 81–87 (1994b).
Kiho, T., Morimoto, H., Sakushima, M., Usui, S., and Ukai, S., Polysaccharides in fungi. XXXV. Anti diabetic activity of an acidic polysaccharide from the fruiting bodies of Tremella aurantia. Biol. Pharm. Bull., 18, 1627–1629 (1995).
Kiho, T., Yamane, A., Hui, J., Usui, S., and Ukai, S., Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol. Pharm. Bull., 19, 294–296 (1996).
Kiho, T., Ookubo, K., Usui, S., Ukai, S., and Hirano, K., Structural features and hypoglycemic activity of a polysaccharide (CS-F10) from the cultured mycelium of Cordyceps sinensis. Biol. Pharm. Bull., 22, 966–970 (1999).
Kiho, T., Morimoto, H., Kobayashi, T., Usui, S., Ukai, S., Aizawa, K., and Inakuma, T., Effect of a polysaccharide (TAP) from the fruiting bodies of Tremella aurantia on glucose metabolism in mouse liver. Biosci. Biotechnol. Biochem., 64, 417–419 (2000).
Kim, Y. W., Kim, K. H., Choi, H. J., and Lee, D. S., Anti-diabetic activity of beta-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett., 27, 483–487 (2005).
Lei, H., Ma, X., and Wu, W. T., Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J. Pharm. Pharmacol., 59, 575–582 (2007).
Li, J. B. and Wassner, S. J., Protein synthesis and degradation in skeletal muscle of chronically uremic rats. Kidney Int., 29, 1136–1143 (1986).
Li, W. L., Zheng, H. C., Bukuru, J., and De Kimpe, N., Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol., 92, 1–21 (2004).
Lindequist, U., Niedermeyer, T. H., and Jülich, W. D., The pharmacological potential of mushrooms. Evid. Based Complement. Alternat. Med., 2, 285–299 (2005).
Liu, Z., Barrett, E. J., Dalkin, A. C., Zwart, A. D., and Chou, J. Y., Effect of acute diabetes on rat hepatic glucose-6-phosphatase activity and its messenger RNA level. Biochem. Biophys. Res. Commun., 205, 680–686 (1994).
Livak, K. J. and Schmittgen, T. D., Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods, 25, 402–408 (2001).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193, 265–275 (1951).
Manohar, V., Talpur, N. A., Echard, B. W., Lieberman, S., and Preuss, H. G., Effects of a water-soluble extract of maitake mushroom on circulating glucose/insulin concentrations in KK mice. Diabetes Obes. Metab., 4, 43–48 (2002).
McCormack, J. G., Westergaard, N., Kristiansen, M., Brand, C. L., and Lau, J., Pharmacological approaches to inhibit endogenous glucose production as a means of antidiabetic therapy. Curr. Pharm. Des., 7, 1451–1474 (2001).
Moller, D. E., New drug targets for type 2 diabetes and the metabolic syndrome. Nature, 414, 821–827 (2001).
Morinaga, H., Yamamoto, H., Sakata, K., Fukuda, S., Ito, M., Sasase, T., Miyajima, K., Ueda, N., Ohta, T., and Matsushita, M., Characterization of hepatic glucose metabolism disorder with the progress of diabetes in male Spontaneously Diabetic Torii rats. J. Vet. Med. Sci., 70, 1239–1245 (2008).
Preuss, H. G., Echard, B., Bagchi, D., Perricone, N. V., and Zhuang, C., Enhanced insulin-hypoglycemic activity in rats consuming a specific glycoprotein extracted from maitake mushroom. Mol. Cell. Biochem., 306, 105–113 (2007).
Rees, D. A. and Alcolado, J. C., Animal models of diabetes mellitus. Diabet. Med., 22, 359–370 (2005).
Paterson, R. R., Ganoderma — a therapeutic fungal biofactory. Phytochemistry, 67, 1985–2001 (2006).
Samuel, V. T., Choi, C. S., Phillips, T. G., Romanelli, A. J., Geisler, J. G., Bhanot, S., McKay, R., Monia, B., Shutter, J. R., Lindberg, R. A., Shulman, G. I., and Veniant, M. M., Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes, 55, 2042–2050 (2006).
Samuel, V. T., Beddow, S. A., Iwasaki, T., Zhang, X. M., Chu, X., Still, C. D., Gerhard, G. S., and Shulman, G. I., Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc. Natl. Acad. Sci. U. S. A., 106, 12121–12126 (2009).
Seto, S. W., Lam, T. Y., Tam, H. L., Au, A. L. S., Chan, S. W., Wu, J. H., Yu, P. H. F., Leung, G. P. H., Ngai, S. M., Yeung, J. H. K., Leung, P. S., Lee, S. M. Y., and Kwan, Y. W., Novel hypoglycemic effects of Ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine, 16, 426–436 (2009).
Shaw, J. E., Sicree, R. A., and Zimmet, P. Z., Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract., 87, 4–14 (2010)
Srinivasan, K., Viswanad, B., Asrat, L., Kaul, C. L., and Ramarao, P., Combination of high-fat diet-fed and low dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res., 52, 313–320 (2005).
Stein, S., Liehr, T., and Eschrich, K., Characterization of the mouse liver fructose-1,6-bisphosphatase gene. Gene, 264, 215–224 (2001).
Stevens, J. F., Determination of glucose by an automatic analyser. Clin. Chim. Acta, 32, 199–201 (1971).
Sun, Y., Liu, S., Ferguson, S., Wang, L., Klepcyk, P., Yun, J. S., and Friedman, J. E., Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J. Biol. Chem., 277, 23301–23307 (2002).
Tomoda, M., Gonda, R., Kasahara, Y., and Hikino, H., Glycan structures of ganoderans b and c, hypoglycemic glycans of ganoderma lucidum fruit bodies. Phytochemistry, 25, 2817–2820 (1986).
Wang, H. M., Huang, S. X., and Sun, W., Study on the hypoglycemic effect and its mechanism of lentinan in alloxaninduced diabetic mice. Chin. J. Nat. Med., 7, 181–184 (2005).
Wright, S. W., Carlo, A. A., Danley, D. E., Hageman, D. L., Karam, G. A., Mansour, M. N., McClure, L. D., Pandit, J., Schulte, G. K., Treadway, J. L., Wang, I. K., and Bauer, P. H., 3-(2-carboxyethyl)-4,6-dichloro-1H-indole-2-carboxylic acid: an allosteric inhibitor of fructose-1,6-bisphosphatase at the AMP site. Bioorg. Med. Chem. Lett., 13, 2055–2058. (2003).
Wu, S. Y., Wang, G. F., Liu, Z. Q., Rao, J. J., Lu, L., Xu, W., Wu, S. G., and Zhang, J. J., Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta. Pharmacol. Sin., 30, 202–208 (2009).
Yuan, Z., He, P., Cui, J., and Takeuchi, H., Hypoglycemic effect of water-soluble polysaccharide from Auricularia auricula-judae Quel. on genetically diabetic KK-Ay mice.. Biosci. Biotechnol. Biochem., 62, 1898–1903 (1998a).
Yuan, Z., He, P., and Takeuchi, H., Ameliorating effects of water-soluble polysaccharides from woody ear (Auricularia auricula-judae Quel.) in genetically diabetic KK-Ay mice. J. Nutr. Sci. Vitaminol. (Tokyo), 44, 829–840 (1998b).
Zhang, J. Y., Zhang, Q. M., Yu, D. M., and Liu, D. M., Effects of metformin on glucose-6-phosphatase expression in diabetic rat liver. Tianjin Med. J., 35, 197–199 (2007).
Zheng, J. G., Gu, Y. J., Yan, S. P., Cai, D. C., Ma, L. G., Mu, D. H., and Li, G. X., Pre-column derivatization and RPHPLC determination of free amino acids in plasma and its application in inborn aminoacidopathies screening. J. Instrumental Anal., 24, 22–25 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xiao, C., Wu, QP., Cai, W. et al. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch. Pharm. Res. 35, 1793–1801 (2012). https://doi.org/10.1007/s12272-012-1012-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-1012-z