Abstract
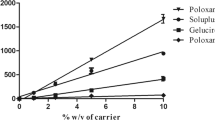
Cryptotanshinone, tanshinone I and tanshinone IIA are three major components in the extract of Salvia miltiorrhiza with pharmacological significance. However, their effective utilization is limited due to poor water solubility and bioavailability. Solid dispersion (SD) of the extract of Salvia miltiorrhiza was prepared to enhance solubility and dissolution of the three major components. Various carriers were screened for SD preparation by conventional solvent method. Dissolution of the components from selected SD systems was compared with commercial tablets of the extract from Salvia miltiorrhiza. The solubility of three components viz., cryptotanshinone, tanshinone I and tanshinone IIA, after forming SD with either of povidone K-30 (PVP K-30) or poloxamer 407, exhibited enhanced solubility in pH 6.8 buffer. Dissolution test revealed that the amount of three components released was higher from SD tablets as compared to the commercial tablets. Pharmacokinetic profile was evaluated using cryptotanshinone as a representative compound. AUC of cryptotanshinone was significantly increased when administered as a solid dispersion.
Similar content being viewed by others
References
Chu, M. Q., Gu, H. C., and Liu, G. J., Stability of solid dispersion of tanshinone. J. East Chin. Univ. Sci. Technol., 28, 107–109 (2002).
Craig, D. Q. M., The mechanism of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm., 231, 131–144 (2002).
Gibaldi, M. and Perrier, D., Pharmacokinetics, 2nd Ed, Marcel Dekker, NY, (1982).
Hu, P., Luo, G. A., Zhao, Z. Z., and Jiang, Z. H., Quality assessment of Radix Salviae Miltiorrhizae. Chem. Pharm. Bull., 53, 481–486 (2005).
Junghanns, J. U. A. H. and Müller, R. H., Nanocrystals technology, drug delivery and clinical applications. Int. J. Nanomed., 3, 295–309 (2008).
Karavas, E., Georgarakis, E., Sigalas, M. P., Avgoustakis, K., and Bikiaris, D., Investigation of the release mechanism of a sparingly water-soluble drug from solid dispersions in hydrophilic carriers based on physical state of drug, particle size distribution and drug-polymer interactions. Eur. J. Pharm. Biopharm., 66, 334–347 (2007).
Keck, C. M. and Müller, R. H., Drug nanocrystals of poorly soluble drugs produced by high pressure homogenization. Eur. J. Pharm. Biopharm., 62, 3–16 (2006).
Kim. K. T., Lee J. Y., Lee, M. Y., Song, C. K., Choi, J., and Kim, D., Solid dispersions as a drug delivery system. J. Pharm. Invest., 41, 125–142 (2011).
Li, J. F., Wei, Y. X., Ding, L. H., and Dong, C., Study on the inclusion complexes of cryptotanshinone with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. Spectrochim. Acta A Mol. Biomol. Spectrosc., 59, 2759–2766 (2003).
Li, X. L., Li, X. R., Wang, L. J., Li, Y. H., Xu, Y. X., and Xue, M., Simultaneous determination of danshensu, ferulic acid, cryptotanshinone and tanshinone IIA in rabbit plasma by HPLC and their pharmacokinetic application in danxiongfang. J. Pharm. Biomed. Anal., 44, 1106–1112 (2007).
Ling, W., Xuehua, J., Weijuan, X., and Chenrui, L., Complexation of tanshinone IIA with 2-hydroxypropyl-β-cyclodextrin: effect on aqueous solubility, dissolution rate, and intestinal absorption behavior in rats. Int. J. Pharm., 341, 58–67 (2007).
Luo, X. and Xu, Y. H., Preparation of cryptotanshinone solid dispersion and its properties. Zhong Cao Yao, 36, 839–842 (2005).
Passerini, N., Gonzalez-Rodriguez, M. L., Cavallari, C., Rodriguez, L., and Albertini, B., Preparation and characterization of ibuprofen-poloxamer 188 granules obtained by melt granulation. Eur. J. Pharm. Sci., 15, 71–78 (2002).
Sethia, S. and Squillante, E., Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int. J. Pharm., 272, 1–10 (2004).
Shi, Z. H., He, J. T., Yao, T. T., Chang, W. B., and Zhao, M. P., Simultaneous determination of cryptotanshinone, tanshinone I and tanshinone IIA in traditional Chinese medicinal preparations containing Radix Salvia Miltiorrhiza by HPLC. J. Pharm. Biomed. Anal., 37, 481–486 (2005).
Song, M., Hang, T. J., Zhang, Z. X., and Chen, H. Y., Effects of the coexisting deterpenoid tanshinones on the pharmacokinetics of cryptotanshinone and tanshinone IIA in rat. Eur. J. Pharm. Sci., 32, 247–253 (2007).
Su, Y. L., Fu, Z. Y., Zhang, J. Y., Wang, W. M., Wang, H., Wang, Y. C., and Zhang, Q. J., Microencapsulation of Radix salvia miltiorrhiza nanoparticles by spray-drying. Powder Technol., 184, 114–121 (2008).
Van den Mooter, G., Augustijns, P., Blaton, N., and Kinget, R., Physico-chemical characterization of solid dispersions of temazepam with polyethylene glycol 6000 and PVP K30. Int. J. Pharm., 164, 67–80 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, H., Subedi, R.K., Nepal, P.R. et al. Enhancement of solubility and dissolution rate of cryptotanshinone, tanshinone I and tanshinone IIA extracted from Salvia miltiorrhiza . Arch. Pharm. Res. 35, 1457–1464 (2012). https://doi.org/10.1007/s12272-012-0816-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-0816-1