Abstract
Purpose
Withania somnifera is a xerophytic plant whose methanolic extract of root powder has exhibited effectiveness against various diseases. Like other natural products, its methanolic root powder extract also displays poor dissolution properties because of high lipophilicity and poor aqueous solubility. The current research was intended to formulate amorphous solid dispersion (ASD) of Withania somnifera root powder extract for improving its dissolution properties and saturation solubility.
Method
The solvent evaporation method was used to prepare the ASD. Among all used polymers, HPMCAS-L, PVP K-30, and Soluplus®, only PVP K-30 at 1:2 and 1:4 ratios was able to create a complete amorphous system with the extract.
Result
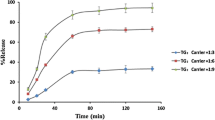
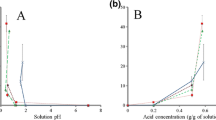
The FTIR spectra indicated no interaction between the phytoconstituents and the HPMCAS-L and Soluplus®. However, hydrogen bonding was observed between the PVP K-30 and phytoconstituents, leading to the formation of a stable amorphous system. All the prepared ASDs had significantly higher saturation solubility than pure extract, but the highest solubility was observed for the PVP K-30–based ASD.
Conclusion
The formed ASDs demonstrated substantial improvement in the dissolution and saturation solubility of phytoconstituent (withanolide A) compared to a pure extract. The ASDs formed with PVP K-30 at 1:2 and 1:4 were able to stabilize the phytoconstituents in the amorphous state at 30 °C/ 65 ± 5 RH for 6 months.







Similar content being viewed by others
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Gupta GL, Rana AC. Withania somnifera (ashwagandha): a review, Pharmacognosy Reviews. 2007;1.
Singh G, Sharma PK, Dudhe R, Singh S. Biological activities of Withania somnifera. Ann Biol Res. 2010;1:56–63.
Kulkarni SK, Dhir A. Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1093–105.
Pawar P, Gilda S, Sharma S, Jagtap S, Paradkar A, Mahadik K, Ranjekar P, Harsulkar A. Rectal gel application of Withania somnifera root extract expounds anti-inflammatory and muco-restorative activity in TNBS-induced inflammatory bowel disease. BMC Complement Altern Med. 2011;11:34.
Rai M, Jogee PS, Agarkar G, Santos CA. Anticancer activities of Withania somnifera: current research, formulations, and future perspectives, Pharmaceut Biol. 2016;54:189–97.
Devi PU, Sharada AC, Solomon FE. Antitumor and radiosensitizing effects of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, sarcoma-180. Indian J Exp Biol. 1993;31:607–11.
Malik F, Singh J, Khajuria A, Suri KA, Satti NK, Singh S, Kaul MK, Kumar A, Bhatia A, Qazi GN. A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007;80:1525–38.
Owais M, Sharad KS, Shehbaz A, Saleemuddin M. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine. 2005;12:229–35.
Chouhan N, Mittal V, Kaushik D, Khatkar A, Raina M. Self emulsifying drug delivery system (SEDDS) for phytoconstituents: a review. Curr Drug Deliv. 2015;12:244–53.
Sayed N, Khurana A, Godugu C. Pharmaceutical perspective on the translational hurdles of phytoconstituents and strategies to overcome. Journal of Drug Delivery Science and Technology. 2019;53:101201.
Chen B, Wang X, Zhang Y, Huang K, Liu H, Xu D, Li S, Liu Q, Huang J, Yao H. Improved solubility, dissolution rate, and oral bioavailability of main biflavonoids from Selaginella doederleinii extract by amorphous solid dispersion. Drug Delivery. 2020;27:309–22.
Wang W, Kang Q, Liu N, Zhang Q, Zhang Y, Li H, Zhao B, Chen Y, Lan Y, Ma Q. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia. 2015;102:189–97.
Weerapol Y, Tubtimsri S, Jansakul C, Sriamornsak P. Improved dissolution of Kaempferia parviflora extract for oral administration by preparing solid dispersion via solvent evaporation. Asian J Pharmaceut Sci. 2017;12:124–33.
Sahoo N, Manchikanti P, Dey S. Herbal drugs: standards and regulation. Fitoterapia. 2010;81:462–71.
Singh LK, Karlo T, Pandey A. Performance of fruit extract of Melastoma malabathricum L. as sensitizer in DSSCs, Spectrochimica Acta Part A: Mol Biomol Spectrosc. 2014;118:938–43.
A. Tomšik, L. Šarić, S. Bertoni, M. Protti, B. Albertini, L. Mercolini, N. Passerini, Encapsulations of wild garlic (Allium ursinum L.) extract using spray congealing technology. Food Res Int 2019;119:941–50.
Dedroog S, Pas T, Vergauwen B, Huygens C, Van den Mooter G. Solid-state analysis of amorphous solid dispersions: why DSC and XRPD may not be regarded as stand-alone techniques. J Pharm Biomed Anal. 2020;178: 112937.
Khan SA, Maher S, Naheed N, Mahmood T, Ayub K, Farooq A, Khan SB, Shah Z. Isolation, spectroscopic and density functional theory of two withanolide glycosides. J Mol Struct. 2019;1177:449–56.
Lloyd GR, Craig DQM, Smith A. A calorimetric investigation into the interaction between paracetamol and polyethlene glycol 4000 in physical mixes and solid dispersions. Eur J Pharm Biopharm. 1999;48:59–65.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Serajuddin ATM. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88:1058–66.
Curatolo W, Nightingale JA, Herbig SM. Utility of hydroxypropylmethylcellulose acetate succinate (HPMCAS) for initiation and maintenance of drug supersaturation in the GI milieu. Pharm Res. 2009;26:1419–31.
Adhikari A, Polli JE. Characterization of grades of HPMCAS spray dried dispersions of itraconazole based on supersaturation kinetics and molecular interactions impacting formulation performance. Pharm Res. 2020;37:1–15.
Alves LD, Soares MF, de Albuquerque CT, da Silva ÉR, Vieira AC, Fontes DA, Figueirêdo CB, Sobrinho JL, Neto PJ. Solid dispersion of efavirenz in PVP K-30 by conventional solvent and kneading methods. Carbohyd Polym. 2014;104:166–74.
Lavra ZMM, Pereira de Santana D, Ré MI. Solubility and dissolution performances of spray-dried solid dispersion of Efavirenz in Soluplus. Drug Dev Ind Pharm. 2017;43:42–54.
Lu J, Cuellar K, Hammer NI, Jo S, Gryczke A, Kolter K, Langley N, Repka MA. Solid-state characterization of Felodipine-Soluplus amorphous solid dispersions. Drug Dev Ind Pharm. 2016;42:485–96.
Shamma RN, Basha M. Soluplus®: a novel polymeric solubilizer for optimization of carvedilol solid dispersions: formulation design and effect of method of preparation. Powder Technol. 2013;237:406–14.
Funding
The DTE, Government of Gujarat, India, provided financial support under the SSIP scheme.
Author information
Authors and Affiliations
Contributions
Kiran Dudhat: main author, practical work. Monika Bhalodiya: analytical work. Ashvin Dudhrejiya: overall guidance. Mori Dhaval: communicating author, concept of research, manuscript preparation.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
No animals or humans were harmed during the course of this research work.
Consent for Publication
The authors agreed to publish the present manuscript in this respected journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dudhat, K., Bhalodiya, M., Dudhrejiya, A. et al. Application of Amorphous Solid Dispersion Technology for Improving the Physicochemical Properties, Saturation Solubility, and In Vitro Dissolution of Withania somnifera Methanolic Root Powder Extract. J Pharm Innov 18, 1338–1349 (2023). https://doi.org/10.1007/s12247-023-09718-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09718-5