Abstract
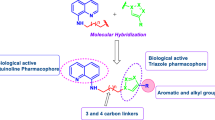
A new series of substituted 1,2,3-triazoles (4a-n) were synthesized from 4-azido-2,8-bistrifluoromethylquinoline 2. The 1,3-dipolar cycloaddition reaction of 2 with ethyl acetoacetate afforded 1-(2,8-Bistrifluoromethylquinolin-4-yl)-5-methyl-1,2,3-triazole-4-carboxylic acid 3, which was then converted into its corresponding acid hydrazide 3a. Condensation of this hydrazide with different aromatic aldehydes resulted in the formation of Schiff’s bases, N-[1-Arylmethylene]-1-[2,8-bistrifluoromethylquinoline-4-yl]-5-methyl-1H-1,2,3-triazole-4-carbohydrazides (4a-n). These newly synthesized 1,2,3-triazole derivatives were characterized by analytical and spectral data. All the synthesized compounds were evaluated in vitro for their antibacterial and antifungal activity. A brief investigation of the structure activity relationships revealed that the nature of the substituent on position 4 of the triazole ring influences the antimicrobial activity. Among the newly synthesized compounds, the most active compound was 4n, which contained the 3-methylthien-2-yl moiety and showed a broad spectrum of antimicrobial activity against all the strains used for testing. Compounds 4b, 4c, 4e, 4f, 4h and 4l showed significant antimicrobial activity at the concentration of 6.25 μg/mL.
Similar content being viewed by others
References
Arthington-Skaggs, B. A., Motley, M., Warnock, D. W., and Morrison, C. J., Comparative evaluation of PASCO and National Committee for clinical laboratory standards M27-A broth micro dilution methods for antifungal drug susceptibility testing of yeasts. J. Clin. Microbiol., 38, 2254–2260 (2000).
Banu, K. M., Dinakar, A., and Ananthanarayanan, C., Synthesis, characterization, antimicrobial screening and pharmacological screening of some substituted 1,2,3-triazoles. Indian J. Pharm. Sci., 16, 202–205 (1999).
Barnard, S., Storr, R. C., O’Neill, P. M., and Park, B. K., The effect of fluorine substitution on the physicochemical properties and the analgesic activity of paracetamol. J. Pharm. Pharmacol., 45, 736–744 (1993).
Barry, A. L., Procedure for testing antimicrobial agents in agar media. Antibiotics in Laboratory Medicine, pp. 1–8, (1991).
Biagi, G., Calderone, V., Giorgi, I., Livi, O., Martinotti, E., Martelli, A., and Naedi, A., 1, 5-Diarylsubstituted 1, 2, 3-triazoles as potassium channel activators. VI. Farmaco, 59, 397–404 (2004).
Bohm, R. and Karow, C., Biologically active triazoles. Pharmazie, 36, 243–247 (1981).
Campos, V. R., Paula, A., A., Helena, C., C., Carlos, R., R., Alessandro, K., J., Vitor, F., F., Maria, C., B., V., de S., Fernanda, da C., S., Laura, A., M., Thaisa, S., D., Carla, C., Eládio, F., S., André, L., F., and Anna, C., C., Synthesis, biological, and theoretical evaluations of new 1,2,3-triazoles. Bioorg. Med. Chem., 17, 7429–7434 (2009).
Davis, R., Markham, A., and Balfour, J. A., Ciprofloxacin, An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugsov, 51, 1019–1074 (1996).
Deng, L., Yang, B., He, Q., and Hu, Y., Novel 1, 2, 3-tiazolelinked norcantharidin analogues: Synthesis and evaluation of growth inhibition in a panel of selected tumor-cell lines. Lett. Drug Des. Discov., 5, 225–231 (2008).
Diana, D. G. and Nitz, J. J., 1,2,4-Oxadiazolyl-phenoxyalkylisoxazoles and their use a s antiviral agents. Eur. Patent, 566199 (1993).
Fenlon, C. H. and Cynamon, M. H., Comparitive in vitro activities of ciprofloxacin and other 4-quinolines against micro bacterium tuberculosis and micro bacterium intracellular, Antimicrob. Agents Chemother., 29, 386–388 (1986).
Guenter, W. P. C. and Vogt, B. R., 3-{Substituted-2-(methylamine) phenyl]-4-[2-oxo-2-(heterocyclic) ethyl]-5-substituted-1,2,4 triazoles. U.S. Patent, 4020064 (1977).
Holla, B. S., Mahalinga, M., Karthikeyan M., S., Boja, Poojary, Akberali, P., M., and Suchetha, Kumari, N., Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur. J. Med. Chem., 40, 1173–1178 (2005).
Julino, M. and Malcolm, S. F. G., Antitumour polycyclic acridines. Part 5. Synthesis of 7H-pyrido[4,3,2-kl]acridines with exploitable functionality in the pyridine ring. J. Chem. Soc., Perkin Trans. I, 1677–1684 (1998).
Karimkulov, K. M., Dzhuraev, A. D., Makhsumov, A. G., and Amanov, N., Synthesis and antimicrobial activity of a number of 1,2,3 triazole derivatives. Pharm. Chem. J., 25, 399–401 (1991).
MacLowry, J. D., Jaqua, M. J., and Selepak, S. T., Detailed methodology and implementation of a semiautomated serial dilution microtechnique for antimicrobial susceptibility testing. Appl. Microbiol., 20, 46–53 (1970).
Manfredini, S., Vicentini, C. B., Manfrini, M., Bianchi, N., Rutigliano, C., Mischiati, C., and Gambari, R., Pyrazolotriazoles as light activable DNA cleaving agents. Bioorg. Med. Chem., 8, 2343–2346 (2000).
Meier, R., Aralkyl triazole compounds. U.S. Patent, 4789680 (1986).
Passannanti, A., Diana, P., Barraja, P., Mingoia, F., Lauria, A., and Cirrincione, G., Pyrrole {2,3-d] triazoles an potential antineoplastic agent. Heterocycles, 48, 1229–1235 (1998).
Sanghvi, Y. S., Bhattacharya, B. K., Kini, C. D., Matsumoto, S. S., Larson, W. B., Jolley, W. B., Robins, R. K., and Revankar, G. R., Growth inhibition and induction of cellular differentiation of human myeloid leukemia cells in culture by carbamoyl congeners of ribavirin. J. Med. Chem., 33, 336–344 (1990).
Savini, L., Massrelli, P., Chiasserini, L., Pellerano, C., and Bruni, G., New 1-[quinolyl(4)]-1,2,3-triazoles: synthesis and evaluation of anti-inflammatory and analgesic properties. Farmaco, 49, 633–639 (1994).
Secor, H. V. and DeBardeleben, J. F., Syntheses and screening of some trifluoromethyl pyrazoles. J. Med. Chem., 14, 997–998 (1971).
Sheremet, E. A., Tomanov, R. I., Trukhin, E. V., and Berestovitsakaya, V. M., Synthesis of 4-aryl-5-nitro-1,2,3-triazoles. Russ. J. Org. Chem., 40, 594–595 (2004).
Verma, R. S., Khan, I. K., and Singh, A. P., Antifungal agents-Past, present and future prospects. National academy of chemistry and biology, Lucknow, India, pp. 29–54, (1998).
Vinaya, K., Kavitha, R., Ananda Kumar, C. S., Benakaprasad, S. B., Chandrappa, S., Deepak, S. A., Nanjundaswamy, S., Umesha, S., and Rangappa, K. S., Synthesis and antimicrobial activity of 1-benzhydryl-sulfonyl-4-(3-(piperidin-4-yl)piperidine derivatives against pathogens of lycopericon esculentum: A structure-activity evaluation study. Arch. Pharm Res., 32, 33–41 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sumangala, V., Poojary, B., Chidananda, N. et al. Synthesis and antimicrobial activity of 1,2,3-triazoles containing quinoline moiety. Arch. Pharm. Res. 33, 1911–1918 (2010). https://doi.org/10.1007/s12272-010-1204-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-1204-3