Abstract
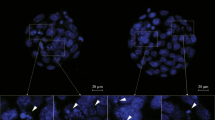
Gangliosides are a family of sialic acid-containing glycosphingolipids that are abundant in neurons and have a variety of functions in developing and mature tissues. We examined the expression of ganglioside GT1b in the embryonic preimplantation stage after freezing and thawing processes to determine the regulatory roles of ganglioside GT1b in early embryonic development. ICR mouse embryos at the two-cell stage obtained by flushing the oviducts were frozen by two cryopreservation procedures, slow freezing using a programmable freezer or vitrification by direct plunging into liquid nitrogen. Slow freezing was conducted with equilibration in 1.5 M 1,2-propanediol or 5% equilibration glycerol. Vitrification was applied with a 10–15 min equilibration in 7.5% ethylene glycol (EG), 7.5% dimethylsulfoxide (DMSO), and 30 sec in a solution of 15% EG, 15% DMSO and 0.5 M sucrose. Immediately after thawing, the survival rate of the embryos was assessed by their morphology and ability to develop to blastocysts in culture. The survival rate of vitrified and thawed embryos (92%) was significantly higher than that of slow frozen and thawed embryos (76%) (P<0.05). A tendency of higher blastocyst rate was found in the vitrified and thawed embryos compared to that of the slow frozen and thawed embryos. Confocal immunofluorescence staining confirmed that surviving embryos expressed ganglioside GT1b, with the strongest expression at the compacted eight-cell or later stage embryos. Ganglioside GT1b was not observed in the TUNEL-positive, apoptotic embryos, suggesting that cryopreservation had induced DNA breaks in them. These results suggest that ganglioside GT1b may play an important role in embryo survival or development.
Similar content being viewed by others
References
Aono, N., Naganuma, T., Abe, Y., Hara, K., Sasada, H., Sato, E., and Yoshida, H., Successful production of blastocysts following ultrarapid vitrification with step-wise equilibriation of germinal vesicle-stage mouse oocytes. J. Reprod. Dev., 49, 501–506 (2003).
Bouvier, J. D. and Seyfired, T. N., Ganglioside composition of normal and mutant mouse embryos. J. Neurochem., 52, 460–466 (1989).
Bremer, E. G., Schlessinger, J., and Hakomori, S., Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J. Biol. Chem., 261, 2434–2440 (1986).
Choo, Y. K., Distribution of ganglioside GM3 in the rat ovary after gonadotropin stimulation. Mol. Cells., 9, 365–375 (1999).
Cooper, A., O’Neil, L., Bernard, A., Fuller, B., and Shaw, R., The effect of protein source and temperature on the damage to mouse oocyte cytoskeleton after exposure to a vitrification solution. Cryo. Lett., 17, 149–156 (1996).
Draper, J. S., Pigott, C., Thomson, J. A., and Andrews, P. W., Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J. Anat., 200, 249–258 (2002).
Dreyfus, H., Urban, P. F., and Edel-Harth, S., Developmental patterns of gangliosides and phospholipids in chick retina and brain. J. Neurochem., 25, 245–250 (1975).
Duchemin, A. M., Ren, Q., Mo, L., Neff, N. H., and Hadjiconstantinou, M., GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem., 81, 696–707 (2002).
Fugger, E. F., Bustillo, M., Katz, L. P., Dorfmann, A. D., Bender, S. D., and Schulman, J. D., Embryonic development and pregnancy from fresh and cryopreserved sibling pronucleate human zygotes.. Fertile. Steril., 50, 273–278 (1998).
Fujino, Y., Ozaki, K., Yamamasu, S., Ito, F., Matsuoka, I., Hayashi, E., Nakamura, H., Ogita, S., Sato, E., and Inoue, M., DNA fragmentation of oocytes in aged mice. Hum. Reprod., 11, 1480–1483 (1996).
Glenister, P. H., Whittingham, D. G., and Wood, M. J., Genome cryopreservation: a valuable contribute to mammalian genetic research. Genet. Res., 56, 564–570 (1990).
Hakomori, S., Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem., 50, 733–764 (1981).
Hakomori, S., Bifunctional role of glycosphingolipids Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem., 265, 18713–18716 (1990).
Hakomori, S. and Igarashi, Y., Functional role of glycosphingolipids in cell recognition and signaling. J. Biochem. (Tokyo), 118, 1091–1103 (1995).
Hanada, K., Nishijima, M., Kiso, M., Hasegawa, A., Fujita, S., Ogawa, T., and Akamatsu, Y., Sphingolipids are essential for the growth of Chinese hamster ovary cells. Restoration of the growth of a mutant defective in sphingoid base biosynthesis by exogenous sphingolipids. J. Biol. Chem., 267, 23527–23533 (1992).
Hidari, K. I., Functional glycoconjugates involved in cellular interaction. Yakugaku Zasshi, 123, 315–322 (2003).
Higashi, H. and Chen, N. H., Ganglioside/protein kinase signals triggering cytoskeletal actin reorganization. Glycoconj. J., 20, 49–58 (2004).
Irwin, L. N., Micheal, D. B., and Irwin, C. C., Ganglioside pattern of fetal rat and mouse brain. J. Neurochem., 34, 1527–1520 (1980).
Ishida, G. M., Saito, H., Ohta, N., Takashi, T., Ito, M. M., Saito, T., Nakahara, K., and Hiroi, M., The optimal equilibration time for mouse embryos frozen by vitrification with trehalose. Hum. Rerpod., 12, 1259–1262 (1997).
Janich, P. and Corbeil, D., GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS. Lett., 581, 1783–1787 (2007).
Jurisicova, A., Varmuza, S., and Casper, R. F., Programmed cell death and human embryo fragmentation. Mol. Hum. Reprod., 2, 93–98 (1996).
Kasai, M., Komi, J. H., Takakamo, A., Tsudera, H., Sakurai, T., and Machida, T., A simple method for mouse embryo cryopreservation in al low toxicity vitrification solution, without appreciable loss of viability. J. Reprod. Fertil., 89, 91–97 (1990).
Kim, S. M., Kwak, D. H., Kim, S. M., Jung, J. U., Lee, D. H., Lee, S., Jung, K. Y., Do, S. I., and Choo, Y. K., Differential expression of gangliosides in the ovary and uterus of streptozotocin-induced and db/db diabetic mice. Arch. Pharm. Res., 29, 666–676 (2006).
Kwak, D. H., Yu, K., Kim, S. M., Lee, D. H., Kim, S. M., Jung, J. U., Seo, J. W., Kim, N., Lee, S., Jung, K. Y., You, H. K., Kim, H. A., and Choo, Y. K., Dynamic changes of gangliosides expression during the differentiation of embryonic and mesenchymal stem cells into neural cells. Exp. Mol. Med., 38, 668–676 (2006).
Lassalle, B., Testart, J., and Remard, J. P., Human embryo features that influence the success of cryopreservation with the use of 1,2 propanediol. Fertil. Sterril., 44, 645–651 (1985).
Lee, D. H., Koo, D. B., Ko, K., Ko, K., Kim, S. M., Jung, J. U., Ryu, J. S., Jin, J. W., Yang, H. J., Do, S. I., Jung, K. Y., and Choo, Y. K., Effects of daunorubicin on ganglioside expression and neuronal differentiation of mouse embryonic stem cells. Biochem. Biophys. Res. Commun., 362, 313–318 (2007).
Leibo, S. P., Mazur, P., and Jackowski, S. J., Factors affecting survival of mouse embryos during freezing and thawing. Exp. Cell. Res., 89, 79–88 (1974).
Li, R., Manela, J., Kong, Y., and Ladisch, S., Cellular gangliosides promote growth factor-induced proliferation of fibroblasts. J. Biol. Chem., 275, 34213–34223 (2000).
Liu, L., Trimarchi, J. R., and Keefe, D. L., Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol. Reprod., 62, 1745–1753 (2000).
Lloyd, K. O. and Furukawa, K., Biosynthesis and functions of gangliosides: recent advances. Glycoconj. J., 15, 627–636 (1998).
Luyet, B. J., The vitrification of organic colloids and protoplasm. Biodynamica., 11–14 (1937).
Maccioni, H. J. F., Panzetta, P., Arrieta, D., and Caputto, R., Ganglioside glycosyltransferase activities in the cerebral hemispheres from developing rat embryos. Int. J. Dev. Neurosci., 2, 13–19 (1984).
Miljan, E. A. and Bremer, E. G., Regulation of growth factor receptors by gangliosides. Sci. STKE., 160, RE15 (2002).
Miyamoto, H. and Ishibashi, T., Survival of frozen-thawed mouse and rat embryos in the presence of ethylene glycol. J. Reprod. Fertil., 50, 373–375 (1977).
Okada, M., Itoh, Mi. M., Haraguchi, M., Okajima, T., Inoue, M., Oishi, H., Matsuda, Y., Iwamoto, T., Kawano, T., Fukumoto, S., Miyazaki, H., Furukawa, K., Aizawa, S., and Furukawa, K., b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem., 277, 1633–1636 (2002).
O’Neil, L., Paynter, S. J., Fuller, B. J., and Shaw, R. W., Murine oocytes cytoskeleton changes, fertilization and embryonic development following exposure to a vitrification solution. Cryo. Lett., 18, 17–26 (1997).
Panzett, P. H. J., Maccioni, H. J., and Caputto, R., Synthesis of retinal gangliosides during chick embryonic development. J. Neurochem., 35, 100–108 (1980).
Rall, W. F. and Fahy, G. M., Ice-free cryopreservation of mouse embryos at-196 degrees C by vitrification. Nature, 313, 573–575 (1985).
Rall, W. F., Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology, 24, 387–402 (1987).
Rosner, H., Ganglioside changes in the chicken optic lobes as biochemical indicators of brain development and maturation. Brain Res., 236, 49–51 (1982).
Selick, C. E., Hofmann, G. E., and Albano, C., Embryo quality and pregnancy potential of fresh compared with frozen embryos freezing detrimental to quality embryos. Hum. Reprod., 10, 392–395 (1995).
Seyfried, T. N., Yu, R. K., Satio, M., and Albert, M. D., Ganglioside abnormalities associated with failed neural differentiation in a T-locus mutant mouse embryo. Dev. Biol., 123, 286–291 (1987).
Sonna, L. A., Fujita, J., Gaffin, S. L., and Lilly, C. M., Molecular biology of thermoregulation invited review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol., 92, 1725–1742 (2002).
Svennerholm, L., Gangliosides and synaptic transmission. Adv. Exp. Med. Biol., 125, 533–544 (1980).
Toledo, M. S., Suzuki, E., Handa, K., and Hakomori, S., Effect of ganglioside and tetraspanins in microdomains on interaction of integrins with fibroblast growth factor receptor. J. Biol. Chem., 280, 16227–16234 (2005).
Uechi, H., Tsutsmi, O., Morita, Y., and Taketani, Y., Cryopreservation of mouse embryos affects later embryonic development possibly through reduced expression of the glucose transporter GLUT1. Mol. Reprod. Dev., 48, 496–500 (1997).
Van Wagtendonk-De Leeuw, A. M., Den Daas, J. H. G., Kruip, A., and Rall, W. F., Comparison of the efficiently of conventional slow freezing and rapid cryopreservation methods for bovine embryos. Cryobiology, 35, 93–105 (1995).
Vancetto, M. D., Curi, L. C., and Pereira, C. A., Neutralization of the effect of Crotalus durissus terrificus venom by gangliosides. Braz. J. Med. Biol. Res., 28, 553–556 (1995).
Whittingham, D. G., Leibo, S. P., and Mazur, P., Survival of mouse embryos frozen to 196 degrees and 169 degrees C. Science, 178, 411–414 (1972).
Wiegandt, H., The chemical constitution of gangliosides of the vertebrate nervous system. Behav. Brain Res., 66, 85–97 (1995).
Yamamoto, H. A. and Mohanan, P. V., Ganglioside GT1B and melatonin inhibit brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Brain Res., 964, 100–106 (2003a).
Yamamoto, H. A. and Mohanan, P. V., In vivo and in vitro effects of melatonin or ganglioside GT1B on L-cysteine-induced brain mitochondrial DNA damage in mice. Toxicol. Sci., 73, 416–422 (2003b).
Yu, R. K., Macala, L. J., Taki, T., Weinfield, H. M., and Yu, F. S., Development changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem., 50, 1825–1829 (1988).
Zurita, A. R., Crespo, P. M., Koritschoner, N. P., and Daniotti, J. L., Membrane distribution of epidermal growth factor receptors in cells expressing different gangliosides. Eur. J. Biochem., 271, 2428–2437 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, BH., Jung, JU., Ko, K. et al. Expression of ganglioside GT1b in mouse embryos at different developmental stages after cryopreservation. Arch. Pharm. Res. 31, 88–95 (2008). https://doi.org/10.1007/s12272-008-1125-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-008-1125-6