Abstract
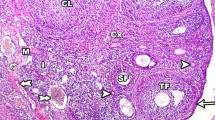
Gangliosides are widely distributed in mammalian cells and play important roles in various functions such as cell differentiation and growth control. In addition, diabetes and obesity cause abnormal development of reproductive processes in a variety of species. However, the mechanisms underlying these effects, and how they are related, are not fully understood. This study examined whether the differential expression of gangliosides is implicated in the abnormal follicular development and uterine architecture of streptozotocin (STZ)-induced and db/db diabetic mice. Based upon the mobility on high-performance thin-layer chromatography, mouse ovary consisted of at least five different ganglioside components, mainly gangliosides GM3, GM1, GD1a and GT1b, and diabetic ovary exhibited a significant reduction in ganglioside expression with apparent changes in the major gangliosides. A prominent immunofluorescence microscopy showed a dramatic loss of ganglioside GD1a expression in the primary, secondary and Graafian follicles of STZ-induced and db/db diabetic mice. A significant decrease in ganglioside GD3 expression was also observed in the ovary of db/db mice. In the uterus of STZ-induced diabetic mice, expression of gangliosides GD1a and GT1b was obviously reduced, but gangliosides GM1, GM2 and GD3 expression was increased. In contrast, the uterus of db/db mice showed a significant increase in gangliosides GM1, GD1a and GD3 expression. Taken together, a complex pattern of ganglioside expression was seen in the ovary and uterus of normoglycemic ICR and db/+ mice, and the correspoding tissues in diabetic mice are characterized by appreciable changes of the major ganglioside expression. These results suggest that alterations in ganglioside expression caused by diabetes mellitus may be implicated in abnormal ovarian development, and uterine structure.
Similar content being viewed by others
References
Baker, L. R., Paddington, A., Goldman, Egler, J., and Moehring, J., Myo-inositol and prostaglandins reverse the glucose inhibition of neutral tube fusion in cultured mouse embryos.Diabetologia, 33, 593–596 (1990).
Becerra, J. E., Khoury, M. J., Cordero, J. F., and Erickson, J. D., Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study.Pediatrics, 85, 1–9 (1990).
Brann, D. W., Wade, M. F., Dhandapani, K. M., Mahesh, V. B., and Buchanan, C. D., Leptin and reproduction.Steroids, 67, 95–104 (2002).
Buchanan, T. A. and Freinkel, N., Fuel-mediated teratogenesis: symmetric growth retardation in the rat fetus at term after a circumscribed exposure to D-mannose during organogenesis.Am. J. Obstet. Gynecol., 158, 663–669 (1988).
Choo, Y. K., Distribution of ganglioside GM3 in the rat ovary after gonadotrophin stimulation.Mol. Cells, 9, 365–375 (1999).
Choo, Y. K., Chiba, K., Tai, T., Ogiso, M., and Hoshi, M., 1995. Differential distribution of gangliosides in adult rat ovary during the oestrous cycle.Glycobiology, 5, 299–309.
Fraser, R., Diabetes in pregnancy.Arch. Dis. Child. Fetal Neonatal, 71, F224-F230 (1994).
Graus, F., Cordon-Cardo, C., Houghton, A. N., Melamed, M. R., and Old, I. J., Distribution of the ganglioside GD3 in human nerve system detected by R24 mouse monoclonal antibody.Brain Res., 324, 190–194 (1984).
Hakomori, S., Glycosphingolipids in cellular interaction, differentiation, and oncogenesis.Annu. Rev. Biochem., 50, 733–764 (1981).
Hakomori, S., Bifunctional role of glycosphingolipids: modulators for transmembrane signaling and mediators for cellular interactions.J. Biol. Chem., 265, 8713–18716 (1990).
Hattori, M. and Horiuchi, R., Biphasic effects of exogenous gnaglioside GM3 on follicle-stimulating hormone-dependent expression of luteinizing hormone receptor in cultured granulosa cells.Mol. Cell Endocrinol., 88, 47–54 (1992).
Horton, Jr. W. E. and Sadler, T. W., Effects of maternal diabetes on early embryogenesis. Alterations in morphogenesis produced by the ketone body, B-hydroxybutyrate.Diabetes, 32, 610–616 (1983).
Ju, E. J., Kwak, D. H., Lee, D. H., Kim, S. M., Kim, J. S., Kim, S. M., Choi, H. G., Jung, K. Y., Lee, S. U., Do, S. I., Park, Y. I., and Choo, Y. K., Pathophysiological implication of Ganglioside GM3 in early mouse embryonic development through apoptosis.Arch. Pharm. Res., 28, 1057–1064 (2005).
Kanai, Y., Kawakami, H., Takata, K., Kurohmaru, M., Hayashi, Y., Nishida, T., and Hirano, H., Localization of Forssman glycolipid and GM1 ganglioside intracellularly and on the surface of germ cells during fetal testicular and ovarian development of mice.Histochemistry, 94, 561–568 (1990).
Kotani, M., Kawashima, I., Ozawa, H., Terashima, T., and Tai, T., Differential distribution of major gangliosides in rat central nerve system detected by specific monoclonal antibodies.Glycobiology, 3, 137–146 (1993).
Kubo, H. and Hoshi, M., Immunohistochemical study of the distribution of a ganglioside in sea urchin eggs.J. Biochem., 108, 193–199 (1990).
Kwak, D. H., Jung, K. Y., Lee, Y. C., and Choo, Y. K., Expressional changes of ganglioside GM3 during ovarian maturation and early embryonic development in db/db mice.Develop. Growth Differ., 45, 95–102 (2003).
Kwak, D. H., Rho, Y. I., Kwon, O. D., Ahn, S. H., Song, J. H., Choo, Y. K., Kim, S. J., Choi, B. K., and Jung, K. Y., Decreases of ganglioside GM3 in streptozotocin-induced diabetic glomeruli of rats.Life Sci., 72, 1997–2006 (2003).
Ledeen, R. W. and Yu, R. K., Gangliosides: structure, isolation, and analysis.Meth. Enzymol., 83, 139–191 (1983).
Seyfried, T. N., Bernard, D. J., and Yu, R. K., 1984. Cellular distribution of gangliosides in the developing mouse cerebellum: analysis using the stagger mutant.J. Neurochem., 43, 1152–1162.
Shogomori, H., Chiba, K., Kubo, H., and Hoshi, M., Non-plasmalemmal localization of the major ganglioside in sea urchin eggs.Zygote, 1, 215–223 (1993).
Sun, G. W., Kobayashi, H., and Terao, T., Expression of link protein during mouse follicular development.J. Histochem. Cytochem., 47, 1433–4442 (1999).
Svennerholm, L., Gangliosides and synaptic transmission. In advances in experimental biology and medicine: structure and function of gangliosides.Adv. Exp. Med. Biol., 125, 533–544 (1980).
Takamatsu, K., Kamei, K., Kubushiro, K., Kiguchi, K., Nozawa, S., and Iwamori, M., Luteal phase-characteristic induction of 13SO3-GalCer in human cervical epithelia, and uterine endometria, and follicular phase-characteristics formation of gangliosides-derived negative charge gradient in different regions of fallopian tubes.Biochim. Biophys. Acta, 1170, 232–236 (1993).
Van Echten, G. and Sandhoff, K., Ganglioside metabolism: enzymology, topology, and regulation.J. Biol. Chem., 268, 5341–5344 (1993).
Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J. M., Positional cloning of the mouse obese gene and its human homologue.Nature, 372, 425–432 (1994).
Zhu, Z., Cheng, L., Tsui, Z., Hakomori, S., and Fenderson, B. A., Glycosphingolipids of rabbit endometrium and their changes during pregnancy.J. Reprod. Fertil., 95, 813–823 (1992).
Zhu, Z., Deng, H., Fenderson, B. A., Nudelman, E. D., and Tsui, Z., Glycosphingolipids of human myometrium and endometrium and their changes during the menstrual cycle, pregnancy and ageing.J. Reprod. Fertil., 88, 71–79 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.M., Kwak, D.H., Kim, S.M. et al. Differential expression of gangliosides in the ovary and uterus of streptozotocin-induced and db/db diabetic mice. Arch Pharm Res 29, 666–676 (2006). https://doi.org/10.1007/BF02968251
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02968251