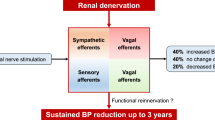
Abstract
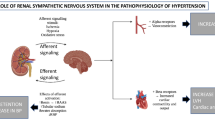
Renal denervation (RDN) is proposed as a durable and patient compliance independent treatment for hypertension. However, 20–30% non-responder after RDN treatment weakened the therapeutic effect, which may be due to blind ablation. The renal nerve mapping/selective ablation system developed by SyMap Medical Ltd (Suzhou), China, has the function of mapping renal sympathetic/parasympathetic nerve sites and selectively removing renal sympathetic nerves and is expected to meet the urgent unmet clinical need of targeted RDN. The “Sympathetic Mapping/Ablation of Renal Nerves Trial” (SMART) is a prospective, multicenter, randomized, single-blinded, sham procedure-controlled trial, to evaluate the safety and efficacy of targeted renal sympathetic denervation in patients with essential and uncontrolled hypertension. The study is the first clinical registry trial using a targeted RDN for the treatment of uncontrolled hypertension; the dual-endpoint design can answer the question of how many antihypertensive drugs can be reduced in patients after RDN. The trial is registered on clinicaltrials.gov NCT02761811.



Similar content being viewed by others
Abbreviations
- ACEI:
-
Angiotensin-converting enzyme inhibition
- AE:
-
Adverse event
- ARB:
-
Angiotensin-II receptor blocker
- BP:
-
Blood pressure
- CCB:
-
Calcium channel blocker
- CTA:
-
Computerized tomographic angiography
- FAS:
-
Full analysis set
- ITT:
-
Intention-to-treat
- LC-M/M:
-
Liquid chromatography with tandem mass spectrometry
- PPS:
-
Per-protocol set
- RDN:
-
Renal denervation
- RF:
-
Radiofrequency
- SAE:
-
Serious adverse event
- SS:
-
Safety set
References
Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–80.
Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–26.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041.
Muntner P, Davis BR, Cushman WC, et al. Treatment-resistant hypertension, and the incidence of cardiovascular disease and endstage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012–21.
de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902.
Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. J Am Med Rec Assoc. 2013;310:959–68.
Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–27.
Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. 2017;390:2549–58.
Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81.
Esler M, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (the SYMPLICITY HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–9.
Murray E. Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8:593–8.
Kandzari DE, Bhate DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219–27.
Kiuchi MG, Esler MD, Fink GD, et al. Renal denervation update from the international sympathetic nervous system summit: JACC State-of-the-Art review. J Am Coll Cardiol. 2019;73:3006–17.
Kandzari DE, Böhm M, Mahfoud F, et al. SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomized trial. Lancet. 2018;391:2346–55.
Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–70.
Bohm M, Kario K, Kandzari D, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomized, sham-controlled trial. Lancet. 2020;395:1444–51.
Townsend RR, Soborka PA. Catheter-based renal denervation for hypertension. Curr Hypertens Rep. 2018;20:93.
Mahfoud F, Renkin J, Sievert H, et al. Alcohol-mediated renal denervation using the peregrine system infusion catheter for treatment of hypertension. JACC Cardiovasc Interv. 2020;13:471–84.
van Amsterdam WA, Blankestijn PJ, Goldschmeding R, et al. The morphological substrate for renal denervation: Nerve distribution patterns and parasympathetic nerves. A post-mortem histological study. Ann Anat. 2016;204:71–9.
Mompeo B, Maranillo E, Garcia-Touchard A, et al. The gross anatomy of the renal sympathetic nerves revisited. Clin Anat. 2016;29:660–4.
Fudim M, Sobotka AA, Yin YH, et al. Selective vs. global renal denervation: a case for less is more. Curr Hypertens Rep. 2018;20:37.
Tan K, Lai Y, Chen W, et al. Selective renal denervation guided by renal nerve stimulation: mapping renal nerves for unmet clinical needs. J Hum Hypertens. 2019;33:716–24.
Liu H, Chen W, Lai Y, et al. Selective renal denervation guided by renal nerve stimulation in canine: a method for identification of optimal ablation target. Hypertension. 2019;74:536–45.
Chinushi M, Izumi D, Kenichi I, et al. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension. 2013;61:450–6.
Chinushi M, Suzuki K, Saitoh O, et al. Electrical stimulation-based evaluation for functional modification of renal autonomic nerve activities induced by catheter ablation. Heart Rhythm. 2016;13:1707–15.
Tsioufis C, Dimitriadis K, Tsioufis P, et al. ConfidenHT™ system for diagnostic mapping of renal nerves. Curr Hypertens Rep. 2018;20:49.
Hilbert S, Kosiuk J, Hindricks G, et al. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Int J Cardiol. 2014;177:669–71.
Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–43.
Lu J, Wang Z, Zhou T, et al. Selective proximal renal denervation guided by autonomic responses evoked via high-frequency stimulation in a preclinical canine model. Circ Cardiovasc Interv. 2015;8:e001847.
Wang J. Mapping sympathetic nerve distribution for renal ablation and catheters for same. US Patent 8702619, published on Dec 15, 2011, and issued on April 22, 2014.
Jamerson KA, Bakris GL, Chuan-Chuan Wun CC, et al. Rationale and design of the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) Trial. Am J Hypertens. 2004;17:793–801.
Hatala R, Pella D, Hatalová K, Sidlo R. Optimization of blood pressure treatment with fixed-combination perindopril/amlodipine in patients with arterial hypertension. Clinical Drug Investig. 2012;32:603–12.
Ma L, Wang W, Zhao Y, Zhang Y, et al. Combination of amlodipine plus angiotensin receptor blocker or diuretics in high-risk hypertensive patients. Am J Cardiovasc Drugs. 2012;12:137–42.
Ma W, Zhang Y, on behalf of HOT-CHINA working group. Low rate of resistant hypertension in Chinese patients with hypertension: an analysis of the HOT-CHINA study. J Hypertens. 2013;31:2386–90.
Kandzari DE, Hickey GL, Pocock SJ, et al. Prioritised endpoints for device-based hypertension trials: the win ratio methodology. EuroIntervention. 2021;16:e1496–502.
Weber MA, Kirtane A, Mauri L, et al. Renal denervation for the treatment of hypertension: making a new start, getting it right. Clin Cardiol. 2015;38:447–54.
Wang Y, Wang JW, Wang Y, et al. Monitoring antihypertensive medication adherence by LC-MS/MS: method establishment and clinical application. J Cardiovasc Pharmacol. 2021;78:581–96.
Kandzari DE, Deepak L, Bhatt D, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITYHTN-3 Trial. Clin Cardiol. 2012;35:528–35.
James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the Eighth Joint National Committee (JNC8). J Am Coll Cardiol. 2014;311:507–20.
Guo Z, Yan X, Yao C. Simulation evaluation of constructing a categorical composite endpoint from two ordered categorical variables. Chin J Health Stat. 2014;31:245–50.
Guo Z, Yao C, Yan X. Overview of constructing methods for the composite endpoint evaluation index in clinical trials. Chinese Journal of New Drugs. 2013;22:2789–830.
Funding
The study is sponsored by SyMap Medical Ltd (Suzhou), China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The protocol was approved by the Ethics Committees of all participated hospitals. All procedures followed are in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent is obtained from all patients previous to being included in the study.
Conflict of Interest
Jie Wang is a co-founder of SyMap Medical Ltd (Suzhou).
Sun N, Jiang H, Yin Y, Chen M, and Huo Y are consultants and received consultant honoraria from SyMap Medical Ltd (Suzhou). Sobotka PA is a consultant and receives consultant honoraria from SyMap Medical Ltd (Suzhou). Yan X received consultant honoraria from SyMap Medical Ltd (Suzhou).
Additional information
Editor-in-Chief Enrique Lara-Pezzi oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Sun, N., Ge, J. et al. Rationale and Design of Sympathetic Mapping/Ablation of Renal Nerves Trial (SMART) for the Treatment of Hypertension: a Prospective, Multicenter, Single-Blind, Randomized and Sham Procedure-Controlled Study. J. of Cardiovasc. Trans. Res. 16, 358–370 (2023). https://doi.org/10.1007/s12265-022-10307-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10307-z