Abstract
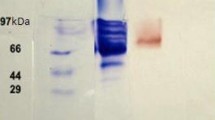
Aryl alcohol oxidase (AAO) produced by dye decolorizing bacteria Sphingobacterium sp. ATM, was purified 22.63 fold to a specific activity of 21.75 μmol/min/mg protein using anion exchange and size exclusion chromatography. The molecular weight of the purified AAO was found to be 71 kDa using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and confirmed by zymography of AAO using L-dopa. The enzyme showed substrate specificity towards veratryl alcohol, followed by n-propanol. The optimum pH and temperature of purified AAO were found to be 3.0 and 40°C, respectively. The K m and V max of AAO was 1.1615 mM and 3.13 mM/min when veratryl alcohol was used as substrate. Sodium azide showed maximum inhibition while ethylenediamine tetra acetic acid (EDTA), L-cysteine and dithiothreitol showed slight inhibition. Metal ions also showed slight inhibition. HPLC analysis confirmed the degradation of Direct Red 5B. The metabolite obtained after decolorization of Direct Red 5B was characterized as 3 diazenyl 7 [-(phenyl carbonyl) amino] naphthalene-2-sulfonic acid using GC-MS analysis.
Similar content being viewed by others
References
Kalme, S., G. Ghodake, and S. Govindwar (2007) Red HE7B degradation using desulfonation by Pseudomonas desmolyticum NCIM 2112. Int. Biodeter. Biodegrade. 60: 327–333.
Dawkar, V., U. Jadhav, S. Jadhav, and S. Govindwar (2008) Biodegradation of disperse textile dye Brown 3REL by newly isolated Bacillus sp. VUS. J. Appl. Microbiol. 105: 14–25.
Jadhav, U., V. Dawkar, G. Ghodake, and S. Govindwar (2008) Biodegradation of Direct Red 5B, a textile dye by newly isolated Comammonas sp. UVS. J. Hazard. Mater. 158: 507–516.
Jadhav, S., S. Kalme, and S. Govindwar (2008) Biodegradation of methyl red by galactomyces geotrichum MTCC 1360. Int. Biodeter. Biodegrade. 62: 135–142.
Telke, A., D. Kalyani, J. Jadhav, and S. Govindwar (2008) Kinetics and mechanism of Reactive Red 141 degradation by a bacterial isolate Rhizobium radiobacter MTCC 8161. Acta Chim. Slov. 55: 320–329.
Kim, S., N. Suzuki, Y. Uematsu, and M. Shoda (2001) Characterization of aryl alcohol oxidase produced by dye-decolorizing fungus, Geotrichum candidum Dec l. J. Biosci. Bioeng. 91: 166–172.
Farmer, V., M. Henderson, and J. Russel (1960) Aromatic-alcohol-oxidase activity in the growth medium of Polystictus versicolor. Biochem. J. 74: 257–262.
Bourbonnais, R. and M. Paice (1988) Veratryl alcohol oxidase from the lignin-degrading basidiomycete Pleurotus sajor-caju. Biochem. J. 255: 445–450.
Guillen, F., A. Martinez, and M. Martinez (1990) Production of hydrogen peroxide by aryl alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Appl. Microbiol. Biotechnol. 32: 465–469.
Sannia, G., P. Limongi, E. Cocca, F. Buonocore, G. Nitti, and P. Giardina (1991) Purification and characterization of a veratryl alcohol oxidase enzyme from the lignin degrading basidiomycete Pleurotus ostreatus. Biochim. Biophys. Acta 1073: 114–119.
Graf, E. and J. Penniston (1980) Method for determination of hydrogen peroxide, with its application illustrated by glucose assay. Clin. Chem. 26: 658–660.
Ander, P. and L. Marzullo (1997) Sugar oxidoreductases and veratrole oxidase as related to lignin degradation. J. Biotechnol. 53: 115–131.
Tamboli, D., A. Kagalkar, M. Jadhav, J. Jadhav, and S. Govindwar (2010) Production of polyhydroxyhexadecanoic waste biomass acid by using of Sphingobacterium sp. ATM generated after degradation of textile dye Direct red 5B. Bioresour. Technol. 101: 2421–2427.
Telke, A., A. kadam, S. Jagtap, J. Jadhav, and S. Givindwar (2010) Biochemical characterization and potential for textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146. Biotechnol. Bioproc. Eng. 15: 696–703.
Tamboli, D., M. Kurade, T. Waghmode, S. Joshi, and S. Govindwar (2010) Exploring the ability of Sphingobacterium sp. ATM to degrade textile dye Direct Blue GLL, mixture of dyes and textile effluent and production of polyhydroxyhexadecanoic acid using waste biomass generated after dye degradation. J. Hazard. Mater. 18: 169–176.
Casieri, L., G. Varese, A. Anastasi, V. prigione, K. Svobodova, V. Filippelo, and C. Novotny (2008) Decolorization and detoxification of reactive industrial dyes by immobilized fungi Trametes pubescens and Pleurotus ostreatus. Folia Microbiol. 53: 44–52.
Guillen, F., A. Martinez, and M. Martinez (1992) Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur. J. Biochem. 209: 603–611.
Varela, E., F. Guillen, A. Martinez, and M. Martinez (2001) Expression of Pleurotus eryngii aryl-alcohol oxidase in Aspergillus nidulans: Purification and characterization of the recombinant enzyme. Biochim. Biophy. Acta 1546: 107–113.
Okamoto, K. and H. Yanase (2002) Aryl alcohol oxidases from the white-rot basidiomycete Pleurotus ostreatus. Mycoscience 43: 391–395.
Kumar, V. and V. Rapheal (2010) Induction and purification by three phase partitioning of aryl alcohol oxidase (AAO) from Pleurotus ostreatus. Appl. Biochem. Biotechnol. 163: 423–432.
Ruiz-Duenas, F., P. Ferreira, M. Martinez, and A. Martinez (2006) In vitro activation, purification, and characterization of Escherichia coli expressed aryl-alcohol oxidase, a unique H2O2-producing enzyme. Prot. Expr. Purif. 45: 191–199.
Ferreira P., M. Medina, F. Guillen, M. Martinez, W. Van Berkel, and A. Martinz (2005) Spectral and catalytic properties of arylalcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem. J. 389: 731–738.
Schwartz, B., A. Olgin, and J. Klinman (2001) The role of cooper in topa quinone biogenesis and catalysis, as probed by azide inhibiton of a copper amine oxidase from yeast. Biochem. 40: 2954–2963.
Shimokawa, T., M. Hirai, M. Shoda, and Y. Sugano (2008) Efficient dye decolorization and production of dye decolorizing enzymes by the basidiomycete Thanatephorus cucumeris Dec 1 in a liquid and solid hybrid culture. J. Biosci. Bioeng. 106: 481–487.
Eichlerova, I., L. Homolka, and F. Nerud (2006) Ability of industrial dyes decolorization and lignolytic enzymes production by different Pleurotus species with special attention on Pleurotus calyptratus, strain CCBAS 461. Proc. Biochem. 41: 941–946.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamboli, D.P., Telke, A.A., Dawkar, V.V. et al. Purification and characterization of bacterial aryl alcohol oxidase from Sphingobacterium sp. ATM and its uses in textile dye decolorization. Biotechnol Bioproc E 16, 661–668 (2011). https://doi.org/10.1007/s12257-011-0031-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-011-0031-9