Abstract
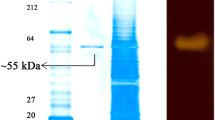
An endosymbiont Halobacterium salinarum MMD047, which could produce high yields of amylase, was isolated from marine sponge Fasciospongia cavernosa, collected from the peninsular coast of India. Maximum production of enzyme was obtained in minimal medium supplemented with 1% sucrose. The enzyme was found to be produced constitutively even in the absence of starch. The optimum temperature and pH for the enzyme production was 40°C and 8.0, respectively. The enzyme exhibited maximum activity in pH range of 6∼10 with an optimum pH of 9.0. The enzyme was stable at 40°C and the enzyme activity decreased dramatically above 50°C. Based on the present findings, the enzyme was characterized as relatively heat sensitive and alkalophilic amylase which can be developed for extensive industrial applications.
Similar content being viewed by others
References
Windish, W. W. and N. S. Mhatre (1965) Microbial amylases. Adv. Appl. Microbiol. 7: 273–304.
Burhan, A., U. Nisa, C. Gokhan, C. Omer, A. Ashabil, and G. Osman (2003) Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem. 38: 1397–1403.
Ali, M. B., M. Mezghani, and S. Bejar (1999) A thermostable α-amylase producing maltohexaose from a new isolated Bacillus sp. US100: study of activity and molecular cloning of the corresponding gene. Enzyme Microb. Technol. 24: 584–589.
Pandey, A., P. Nigam, C. R. Soccol, V. T. Soccol, D. Singh, and R. Mohan (2000) Advances in microbial amylases. Biotechnol. Appl. Biochem. 31: 135–152.
Chandrasekaran, M. (1997) Industrial enzymes from marine microorganisms: the Indian scenario. J. Mar. Biotechnol. 5: 86–89.
Ramachandran, S., A. K. Patel, K. M. Nampoothiri, F. Francis, V. Nagy, G. Szakacs, and A. Pandey (2004) Coconut oil cake — A potential raw material for the production of α-amylase. Bioresour. Technol. 93: 169–174.
Babu, K. R. and T. Satyanarayana (1993) Extracellular calcium-inhibited alpha-amylase of Bacillus coagulans B 49. Enzyme Microb. Technol. 15: 1066–1069.
Mc Tigue, M. A., C. T. Kelly, E. M. Doyle, and W. M. Fogarty (1995) The alkaline amylase of the alkalophilic Bacillus sp. IMD 370. Enzyme Microb. Technol. 17: 570–573.
Gupta, R., P. Gigras, H. Mohapatra, V. K. Goswami, and B. Chauhan (2003) Microbial α-amylases: a biotechno logical perspective. Process Biochem. 38: 1599–1616.
Wanderley, K. J., F. A. G. Torres, L. M. P. Moraes, and C. J. Ulhoa (2004) Biochemical characterization of α-amylase from the yeast Cryptococcus flavus. FEMS Microbiol. Lett. 231: 165–169.
Cappucino, J. G. and N. Sherman (2004) Microbiology: A Laboratory Manual. pp. 491. Pearson Education, Singapore.
Collins, C. H., P. M. Lyne, and J. M. Grange (1989) Microbiological Methods. Butterworths, London, UK.
Enticknap, J. J., M. Kelly, O. Peraud, and R. T. Hill (2006) Characterization of a culturable alphaproteobacterial symbiont common to many marine sponges and evidence for vertical transmission via sponge larvae. Appl. Environ. Microbiol. 72: 3724–3732.
Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman (1990) Basic local alignment search tool. J. Mol. Biol. 215: 403–410.
Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402.
Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Bernfeld, P. (1955) Amylases, α and β. Methods Enzymol. 1: 149–158.
Miller, G. L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428.
George, V. and A. M. Diwan (1993) Simultaneous staining of proteins during polyacrylamide gel electrophoresis in acidic gels by counter migration of Coomasie brilliant blue R-250. Anal. Bacteriol. 4: 662–666.
Anto, H., U. Trivedi, and K. Patel (2006) Alpha amylase production by Bacillus cereus MTCC 1305 using solidstate fermentation. Food Technol. Biotechnol. 44: 241–245.
Asgher, M., M. J. Asad, S. U. Rahman, and R. L. Legge (2007) A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. J. Food Eng. 79: 950–955.
Bajpai, P. and P. K. Bajpai (1989) High-temperature alkaline α-amylase from Bacillus licheniformis TCRDC-B13. Biotechnol. Bioeng. 33: 72–78.
Meers, J. L. (1972) The regulation of α-amylase production in Bacillus licheniformis. Antonie Van Leeuwenhoek 38: 585–590.
Nyiri, L. (1971) The preparation of enzyme by fermentation. Int. J. Chem. Eng. 11: 447–457.
Dettori-Campus, B. G., F. G. Priest, and J. R. Stark (1992) Hydrolysis of starch granules by the amylase from Bacillus stearothermophilus NCA 26. Process Biochem. 27: 17–21.
Nguyen, Q. D., J. M. Rezessy-Szabo, and A. Hoschke (2000) Optimisation of composition of media for the production of amylolytic enzymes by Thermomyces lanuginosus ATCC 34626. Food Technol. Biotechnol. 38: 229–234.
Pedersen, H. and J. Nielsen (2000) The influence of nitrogen sources on the α-amylase productivity of Aspergillus oryzae in continuous cultures. Appl. Microbiol. Biotechnol. 53: 278–281.
Fogarty, W. (1983) Microbial Enzymes and Biotechnology. Applied Science Publishers, London and New York.
Chandra, A. K., S. Medda, and A. K. Bhadra (1980) Production of extracellular thermostable α-amylase by Bacillus licheniformis. J. Ferment. Technol. 58: 1–10.
Saito, N. and K. Yamamoto (1975) Regulatory factors affecting α-amylase production in Bacillus licheniformis. J. Bacteriol. 121: 848–856.
Lin, L. L., C. C. Chyau, and W. H. Hsu (1998) Production and properties of a raw-starch-degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol. Appl. Biochem. 28: 61–68.
Zhang, J. W. and R. Y. Zeng (2008) Purification and characterization of a cold-adapted α-amylase produced by Nocardiopsis sp. 7326 isolated from Prydz Bay, Antarctic. Mar. Biotechnol. 10: 75–82.
Grant, W. D. and K. Horikoshi (1989) Microbiology of extreme environments and its potential for biotechnology. pp. 346–366. In: M. S. Da Costa, J. C. Duarte, and R. A. D. Williams (eds.). Alkaliphiles. Elsevier Applied Science, London, UK.
Nakai, R., T. Saito, and K. Okamoto (1986) Manufacture of alkaline amylase with Streptomyces. Japanese Kokai Koho Patent 86,209.
Ozaki, A. and A. Tanaka (1990) Heat stable alkaline amylase from Bacillus. Japanese Kokai Koho Patent 9049,584.
Wilson, J. J. and W. M. Ingledew (1982) Isolation and characterization of Schwanniomyces alluvius amylolytic enzymes. Appl. Environ. Microbiol. 44: 301–307.
Cordeiro, C. A. M., M. L. L. Martins, and A. B. Luciano (2002) Production and properties of α-amylase from thermophilic Bacillus sp. Braz. J. Microbiol. 33: 57–61.
Igarashi, K., Y. Hatada, H. Hagihara, K. Saeki, M. Takaiwa, T. Uemura, K. Ara, K. Ozaki, S. Kawai, T. Kobayashi, and S. Ito (1998) Enzymatic properties of a novel liquefying alpha-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Appl. Environ. Microbiol. 64: 3282–3289.
Sarikaya, E. and V. Gurgun (2000) Increase of the α-amylase yield by some Bacillus strains. Turk. J. Biol. 24: 299–308.
Amoozegar, M. A., F. Malekzadeh, and K. A. Malik (2003) Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J. Microbiol. Methods 52: 353–359.
Coronado, M. J., C. Vargas, J. Hofemeister, A. Ventosa, and J. J. Nieto (2000) Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol. Lett. 183: 67–71.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found online at http://dx.doi.org/10.1007/s12257-009-1003-1.
Rights and permissions
About this article
Cite this article
Shanmughapriya, S., Seghal Kiran, G., Selvin, J. et al. Optimization, production, and partial characterization of an alkalophilic amylase produced by sponge associated marine bacterium Halobacterium salinarum MMD047. Biotechnol Bioproc E 14, 67–75 (2009). https://doi.org/10.1007/s12257-008-0060-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-008-0060-1