Abstract
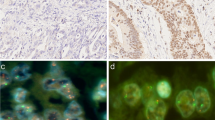
To correlate H2Bub1 expression with outcome in colorectal cancer, H2Bub1 expression was analyzed by immunohistochemistry on a tissue microarray containing 1800 colorectal cancers. Results were compared to clinicopathological parameters.
H2Bub1 IHC was seen in 1256 (79.3 %) of 1584 interpretable CRC and was considered weak in 26.2 % and strong in 53.1 % of cancers. H2Bub1 expression was completely lost in 20.7 % of the cases. Loss of H2Bub1 expression was associated with high tumor grade (p = 0.0211), high tumor stage (p = 0.0003), positive nodal status (p = 0.0139) and histological tumor type (p = 0.0202). No link was found between H2Bub1 expression and tumor localization (p = 0.1262), peritumoral lymphocytic infiltration (p = 0.2523) or vascular invasion (p = 0.5970).
Loss of H2Bub1 expression in CRC was strongly associated with poor patient survival (p = 0.0006). This observation held true also in a subset survival analysis of nodal negative (N0) and nodal positive (N1) cancers (p = 0.0296 and p = 0.0197, respectively). In the subgroup of p53 negative cancers no prognostic impact of H2Bub1 staining was seen (p = 0.1924), whereas in p53 positive CRC H2Bub1 expression loss was associated with poor prognosis (p = 0.0031). Strikingly worsened outcome was found for nodal negative cancers presenting with accumulation of p53 when H2Bub1 expression was lost (p = 0.0006).
Our data demonstrate that a reduced H2Bub1 expression is a strong prognostic biomarker both in nodal negative and nodal positive CRC. H2Bub1 expression measurement might help to select nodal negative CRC patients that may benefit from adjuvant therapy.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Bilchik AJ, Nora DT, Saha S, Turner R, Wiese D, Kuo C, Ye X, Morton DL, Hoon DS (2002) The use of molecular profiling of early colorectal cancer to predict micrometastases. Arch Surg 137(12):1377–1383
Kim J, Hake SB, Roeder RG (2005) The human homolog of yeast BRE1 functions as a transcriptional coactivator through direct activator interactions. Mol Cell 20(5):759–770. doi:10.1016/j.molcel.2005.11.012
Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, Reinberg D (2006) Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell 125(4):703–717. doi:10.1016/j.cell.2006.04.029
Minsky N, Shema E, Field Y, Schuster M, Segal E, Oren M (2008) Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol 10(4):483–488. doi:10.1038/ncb1712
Johnsen SA (2012) The enigmatic role of H2Bub1 in cancer. FEBS Lett 586(11):1592–1601. doi:10.1016/j.febslet.2012.04.002
Pirngruber J, Shchebet A, Schreiber L, Shema E, Minsky N, Chapman RD, Eick D, Aylon Y, Oren M, Johnsen SA (2009) CDK9 directs H2B monoubiquitination and controls replication-dependent histone mRNA 3′-end processing. EMBO Rep 10(8):894–900. doi:10.1038/embor.2009.108
Kari V, Shchebet A, Neumann H, Johnsen SA (2011) The H2B ubiquitin ligase RNF40 cooperates with SUPT16H to induce dynamic changes in chromatin structure during DNA double-strand break repair. Cell Cycle 10(20):3495–3504. doi:10.4161/cc.10.20.17769
Moyal L, Lerenthal Y, Gana-Weisz M, Mass G, So S, Wang SY, Eppink B, Chung YM, Shalev G, Shema E, Shkedy D, Smorodinsky NI, van Vliet N, Kuster B, Mann M, Ciechanover A, Dahm-Daphi J, Kanaar R, Hu MC, Chen DJ, Oren M, Shiloh Y (2011) Requirement of ATM-dependent monoubiquitylation of histone H2B for timely repair of DNA double-strand breaks. Mol Cell 41(5):529–542. doi:10.1016/j.molcel.2011.02.015
Karpiuk O, Najafova Z, Kramer F, Hennion M, Galonska C, Konig A, Snaidero N, Vogel T, Shchebet A, Begus-Nahrmann Y, Kassem M, Simons M, Shcherbata H, Beissbarth T, Johnsen SA (2012) The histone H2B monoubiquitination regulatory pathway is required for differentiation of multipotent stem cells. Mol Cell 46(5):705–713. doi:10.1016/j.molcel.2012.05.022
Trujillo KM, Osley MA (2012) A role for H2B ubiquitylation in DNA replication. Mol Cell 48(5):734–746. doi:10.1016/j.molcel.2012.09.019
Sadeghi L, Siggens L, Svensson JP, Ekwall K (2014) Centromeric histone H2B monoubiquitination promotes noncoding transcription and chromatin integrity. Nat Struct Mol Biol 21(3):236–243. doi:10.1038/nsmb.2776
Espinosa JM (2008) Histone H2B ubiquitination: the cancer connection. Genes Dev 22(20):2743–2749. doi:10.1101/gad.1732108
Prenzel T, Begus-Nahrmann Y, Kramer F, Hennion M, Hsu C, Gorsler T, Hintermair C, Eick D, Kremmer E, Simons M, Beissbarth T, Johnsen SA (2011) Estrogen-dependent gene transcription in human breast cancer cells relies upon proteasome-dependent monoubiquitination of histone H2B. Cancer Res 71(17):5739–5753. doi:10.1158/0008-5472.CAN-11-1896
Hahn MA, Dickson KA, Jackson S, Clarkson A, Gill AJ, Marsh DJ (2012) The tumor suppressor CDC73 interacts with the ring finger proteins RNF20 and RNF40 and is required for the maintenance of histone 2B monoubiquitination. Hum Mol Genet 21(3):559–568. doi:10.1093/hmg/ddr490
Urasaki Y, Heath L, Xu CW (2012) Coupling of glucose deprivation with impaired histone H2B monoubiquitination in tumors. PLoS One 7 (5):e36775. doi:10.1371/journal.pone.0036775
Mirlacher M, Simon R (2010) Recipient block TMA technique. Methods Mol Biol 664:37–44. doi: 10.1007/978-1-60761-806-5_4
Marx A, Simon P, Simon R, Mirlacher M, Izbicki JR, Yekebas E, Kaifi JT, Terracciano L, Sauter G (2008) AMACR expression in colorectal cancer is associated with left-sided tumor localization. Virchows Archiv : An Int J Pathol 453(3):243–248. doi:10.1007/s00428-008-0646-1
Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih Ie M, Kurman RJ (2011) Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Modern Pathology : an Official Journal of the United States and Canadian Academy of Pathology, Inc 24(9):1248–1253. doi:10.1038/modpathol.2011.85
Zhang F, Yu X (2011) WAC, a functional partner of RNF20/40, regulates histone H2B ubiquitination and gene transcription. Mol Cell 41(4):384–397. doi:10.1016/j.molcel.2011.01.024
Chernikova SB, Razorenova OV, Higgins JP, Sishc BJ, Nicolau M, Dorth JA, Chernikova DA, Kwok S, Brooks JD, Bailey SM, Game JC, Brown JM (2012) Deficiency in mammalian histone H2B ubiquitin ligase Bre1 (Rnf20/Rnf40) leads to replication stress and chromosomal instability. Cancer Res 72(8):2111–2119. doi:10.1158/0008-5472.CAN-11-2209
Nakamura K, Kato A, Kobayashi J, Yanagihara H, Sakamoto S, Oliveira DV, Shimada M, Tauchi H, Suzuki H, Tashiro S, Zou L, Komatsu K (2011) Regulation of homologous recombination by RNF20-dependent H2B ubiquitination. Mol Cell 41(5):515–528. doi:10.1016/j.molcel.2011.02.002
Shema E, Tirosh I, Aylon Y, Huang J, Ye C, Moskovits N, Raver-Shapira N, Minsky N, Pirngruber J, Tarcic G, Hublarova P, Moyal L, Gana-Weisz M, Shiloh Y, Yarden Y, Johnsen SA, Vojtesek B, Berger SL, Oren M (2008) The histone H2B-specific ubiquitin ligase RNF20/hBRE1 acts as a putative tumor suppressor through selective regulation of gene expression. Genes Dev 22(19):2664–2676. doi:10.1101/gad.1703008
Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C, Hieter P (2008) Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A 105(9):3443–3448. doi:10.1073/pnas.0712384105
Chernikova SB, Dorth JA, Razorenova OV, Game JC, Brown JM (2010) Deficiency in Bre1 impairs homologous recombination repair and cell cycle checkpoint response to radiation damage in mammalian cells. Radiat Res 174(5):558–565. doi:10.1667/RR2184.1
Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, vanTuinen P, Ledbetter DH, Barker DF, Nakamura Y, White R, Vogelstein B (1989) Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 244(4901):217–221
Rinaldo C, Moncada A, Gradi A, Ciuffini L, D’Eliseo D, Siepi F, Prodosmo A, Giorgi A, Pierantoni GM, Trapasso F, Guarguaglini G, Bartolazzi A, Cundari E, Schinina ME, Fusco A, Soddu S (2012) HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody. Mol Cell 47(1):87–98. doi:10.1016/j.molcel.2012.04.029
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nathaniel Melling and Norbert Grimm contributed equally to this study.
Rights and permissions
About this article
Cite this article
Melling, N., Grimm, N., Simon, R. et al. Loss of H2Bub1 Expression is Linked to Poor Prognosis in Nodal Negative Colorectal Cancers. Pathol. Oncol. Res. 22, 95–102 (2016). https://doi.org/10.1007/s12253-015-9977-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-015-9977-9