Abstract
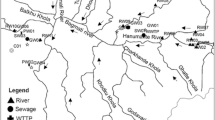
Tobacco mosaic virus (TMV) causes significant yield loss in susceptible crops irrigated with contaminated water. However, detection of TMV in water is difficult owing to extremely low concentrations of the virus. Here, we developed a simple method for the detection and quantification of TMV in irrigation water. TMV was reliably detected at concentrations as low as 10 viral copies/μL with real-time PCR. The sensitivity of detection was further improved using polyethylene glycol 6000 (PEG6000, MW 6000) to concentrate TMV from water samples. Among the 28 samples from Shaanxi Province examined with our method, 17 were tested positive after virus concentration. Infectivity of TMV in the original water sample as well as after concentration was confirmed using PCR. The limiting concentration of TMV in water to re-infect plants was determined as 102 viral copies/mL. The method developed in this study offers a novel approach to detect TMV in irrigation water, and may provide an effective tool to control crop infection.
Similar content being viewed by others
References
Abbaszadegan M, Huber M S, Gerba C P, Pepper I L. 1993. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol, 59: 1213–1219.
Agindotan B O, Shiel P J, Berger P H. 2007. Simultaneous detection of potato viruses, PLRV, PVA, PVX and PVY from dormant potato tubers by TaqMan®real-time RT-PCR. J Virol Methods, 142: 1–9.
Bar-Joseph M, Salomon R. 1980. Heterologous reactivity of tobacco mosaic virus strains in enzyme-linked immunosorbent assays. J Gen Virol, 47: 509–512.
Bertolini E, Moreno A, Capote N, Olmos A, de Luis A, Vidal E, Perez-Panades J, Cambra M. 2008. Quantitative detection of citrus tristeza virus in plant tissues and single aphids by realtime RT-PCR. Eur J Plant Pathol, 120: 177–188.
Boben J, Kramberger P, Petrovic N, Cankar K, Peterka M, Strancar A, Ravnikar M. 2007. Detection and quantification of tomato mosaic virus in irrigation waters. Eur J Plant Pathol, 118: 59–71.
Chen R, Zhu X, Wang Z, Guo Z, Dong H, Wang L, Liu Y, Shi J. 1997. A report of investigating and studying tobacco infectious diseases of 16 main tobacco producing provinces (regions) in China. ChinTob Sci, 4: 1–7.
Chun P W, Fried M, Ellis E F. 1967. Use of water-soluble polymers for the isolation and purification of human immunoglobulins. Anal Biochem, 19: 481–497.
Colombet J, Robin A, Lavie L, Bettarel Y, Cauchie H M, Sime-Ngando T. 2007. Virioplankton ‘pegylation’: use of PEG (polyethylene glycol) to concentrate and purify viruses in pelagic ecosystems. J Microbiol Methods, 71: 212–219
Deboosere N, Horm S V, Pinon A, Gachet J. 2011. Development and validation of a concentration method for the detection of infl uenza A viruses from large volumes of surface water. Appl Environ Microbiol, 77: 3802–3808.
Gooding G V J, Hebert T T. 1967. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology, 57: 1285–1285.
He M, He C Q, Ding N Z. 2012. Natural recombination between tobacco and tomato mosaic viruses. Virus Res, 163: 374–379.
Jacobi V, Bachand G, Hamelin R, Castello J. 1998. Development of a multiplex immunocapture RT-PCR assay for detection and differentiation of tomato and tobacco mosaic tobamoviruses. J Virol Methods, 74: 167–178.
Jaykus L A, De-Leon R, Sobsey M D. 1996. A virion concentration method for detection of human enteric viruses in oysters by PCR and oligoprobe hybridization. Appl Environ Microbiol, 62: 2074–2080.
Jiang X, Wang J, Graham D Y, Estes M K. 1992. Detection of norwalk virus in stool by polymerase chain reaction. J Clin Microbiol, 30: 2529–2534.
Li J W, Wang X W, Rui Q Y, Song N, Zhang F G, Ou Y C, Chao F H. 1998. A new and simple method for concentration of enteric viruses from water. J Virol Methods, 74: 99–108.
Liu Y, Wang Z, Qian Y, Mu J, Shen L, Wang F, Yang J. 2010. Rapid detection of tobacco mosaic virus using the reverse transcription loop-mediated isothermal amplification method. Arch Virol, 155: 1681–1685.
Philipson L, Albertsson P A, Frick G. 1960. The purifi cation and concentration of viruses by aqueous polymer phase systems. Virology, 11: 553–571.
Rowhani A, Maningas M, Lile L, Daubert S, Golino D. 1995. Development of a detection system for viruses of woody plants based on PCR analysis of immobilized virions. Phytopathology, 85: 347–352.
Schwab K J, De-Leon R, Sobsey M D. 1996. Immunoaffinity concentration and purifi cation of waterborne enteric viruses for detection by reverse transcriptase PCR. Appl Environ Microbiol, 62: 2086–2094.
Shieh Y S C, Wait D, Tai L, Sobsey M D. 1995. Methods to remove inhibitors in sewage and other fecal wastes for entero virus detection by polymerase chain reaction. J Virol Methods, 54: 51–66.
Tosic M, Tosic D. 1984. Occurrence of tobacco mosaic virus in water of the Danube and Sava rivers. J phytopathologyphytopathologische zeitschrift, 110: 200–202.
Wetzel T, Candresse T, Macquaire G, Ravelonandro M, Dunez J. 1992. A highly sensitive immunocapture polymerase chain reaction method for plum pox potyvirus detection. J Virol Methods, 39: 27–37.
Yang F, Xu X. 1993. A new method of RNA preparation for detection of hepatitis A virus in environmental samples by the polymerase chain reaction. J Virol Methods, 43: 77–84.
Zheng Y T, Lin Q Y, Xie L H. 2000. Plant viruses and their ecological in water. Virologica sinica, 15: 1–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Liu, W., Jiao, H. et al. Development of a concentration method for detection of tobacco mosaic virus in irrigation water. Virol. Sin. 29, 155–161 (2014). https://doi.org/10.1007/s12250-014-3461-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-014-3461-7