Abstract
Tomato spotted wilt virus (TSWV) is considered one of the most threatening viruses worldwide for different economically important agricultural crops. In this scenario, it is important to perform an early detection by laboratory tests to prevent TSWV spread. A rapid and sensitive TSWV detection protocol based on real time reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay was developed in this work, also using cost-effective and simplified sample preparation procedure, to assess the suitability of the RT-LAMP assay in field conditions on tomato and pepper samples. A set of six primers was designed within the nucleotide sequence region coding for the nucleocapsid protein (N) of segment S, targeting a 220-nucleotide sequence. Sensitivity, specificity, accuracy, and in-field application of the real-time RT-LAMP assay were evaluated. The developed real-time RT-LAMP assay proved to be one thousand and one hundred times more sensitive than end-point RT-PCR and real-time RT-PCR methods, respectively, detecting a total of 9.191 × 101 genome copies as minimum target, and no cross-reactivity were detected with other viruses belonging to Tospoviridae and Bromoviridae families used as outgroup. In addition, the in-field application of the assay using the rapid sample preparation gave adequate and reliable results within 60 minutes, with an acceptable reaction delay when compared to canonical RNA extraction. The in-field analyses showed an increase of TSWV-positive samples (37%) detection compared with end-point RT-PCR and real-time RT-PCR (32% and 29%, respectively), particularly on asymptomatic samples, confirming that the real-time RT-LAMP assay can be implemented as a routine test both in-field and laboratory conditions as a rapid and sensitive technique for TSWV detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato spotted wilt virus (TSWV), assigned to the species Orthotospovirus tomatomaculae, belonging to the Orthotospovirus genus (Tospoviridae family), is considered one of the most threatening viruses worldwide, as its host range includes economically important crops and ornamental plants in temperate, subtropical, and tropical regions (Sialer et al. 2000; Davino et al. 2004). TSWV has a tripartite genome consisting of large (L), medium (M) and small (S) single-stranded RNA segments (Kormelink et al. 2011). The negative-sense L RNA segment (8.9 kb) encodes for RNA-dependent RNA polymerase (RdRp), which is necessary for viral RNA replication and mRNA transcription. (De Haan et al. 1991; Adkins et al. 1995). The ambisense M RNA segment (4.8 kb) has two open reading frames (ORFs) coding for a nonstructural protein (NSm) and for protein precursor of glycoproteins (GN and GC). The NSm protein is involved in cell-to-cell movement of the virus (Kormelink et al. 1994), whereas the glycoproteins act as viral attachment proteins (Bandla et al. 1998). The ambisense S RNA segment (2.9 kb) encodes for a nonstructural protein (NSs) and for the nucleo-capsid protein (N) (De Haan et al. 1990). The NSs protein is an RNA silencing suppressor (Takeda et al. 2002), and the N protein forms the virus nucleocapsid (Richmond et al. 1998). The disease was first described in Australia in 1915 (Brittlebank 1919) and only subsequently was demonstrated to be a viral agent (Samuel et al. 1930). Afterward, the disease has been recorded in other regions of Europe, South America, North America, Africa, and Asia; to date, it is a widely distributed pathogen worldwide.
Usually, the impact on tomato crop losses caused by TSWV can reach values that exceed 40%, with over 90% of reduction in quality and marketable tomatoes (Sevik and Arli-Sokmen 2012) and worldwide losses that overcome one billion dollars annually (Saidi and Warade 2008). In addition to tomato crop, other important horticultural species with great losses caused by TSWV infection are pepper, lettuce and eggplant (Roselló et al. 1996).
Tomato spotted wilt virus is included in the OEPP/EPPO A2 pests list recommended for regulation as a quarantine pathogen (EPPO 2023a). In Italy, TSWV was first reported in 1989 in northern areas (Bellardi and Vicchi 1990), and subsequently its presence was recorded across central and southern regions (Triolo et al. 1991; Davino et al. 1992). Compared to other plant viruses, its host range is broader and includes over 900 plants species, among monocotyledons and dicotyledons, across more than 90 families (Pappu 2008). Major hosts include Solanaceae plants, such as tomatoes, peppers, eggplant and tobacco, for which TSWV is a main production constraint worldwide (Pappu 2008).
The symptom diversity depends on the host species and cultivar, plant stage, crop season and environmental conditions (Oliver and Whitfield 2016). Susceptibility and symptom expression in the host, as well as virus transmission, replication and translocation, are greatly affected by temperature and photoperiod; in fact, it was observed that TSWV replication was greatest at 20 °C, while symptoms were most severe at 33–36 °C (Llamas-Llamas et al. 1998; Moury et al. 1998). In general, TSWV symptoms are various and include chlorosis, ring spots, mottling, silvering, necrosis of different plant parts, stunted growth, local lesions and even plant death in case of severe infections (Pappu 2008). In particular, the initial symptoms on tomato leaves can be confused with cold damage, as the symptoms observed on the youngest leaves consist of violet-brown discolorations to the under-leaf side, known as “leaf bronzing” (Davino et al. 2018), subsequently, typical symptoms evolve into small brown spots, stunted growth and decay of the apices. In addition, necrotic and concentric rings often appear on growing tomato fruit, which turn yellow on ripe fruits (Roselló et al. 1996). On pepper plants, TSWV causes severe stunting of the growth of young plants and a chlorotic mosaic or yellow spot on the leaves; infections on mature plants cause chlorotic lines with necrotic spots that are also observed on fruits, which often show a ring pattern (Davino et al. 2018).
TSWV is naturally transmitted by at least 10 thrips species (Thysanoptera: Thripidae), belonging to Frankliniella and Thrips genera (Cho et al. 1987; Mound 1995; Moyer 2000; Ullman et al. 2002), both in a circulative and propagative manner (Ullman et al. 1993; Wijkamp et al. 1993). TSWV began to be a major threat to European horticulture in the 1980 (Smith et al. 1992), when Frankliniella occidentalis (Pergande) was first reported in northern Italy in 1987 (Rampinini 1987). Moreover, it has been reported that the virus can also be transmitted by seed and pollen (Wang et al. 2022), while it cannot be mechanically transmitted, except under laboratory conditions (Krishna-Kumar et al. 1993).
Like other bunyaviruses, TSWV have a great potential for rapid evolution easily overcomes resistance genes, leading to the emergence of new resistance-breaking (RB) strains, causing severe economic losses in agricultural crops that commonly amount up to 50% (Crescenzi et al. 2015). Due to the large number of TSWV strains and the facility to recombine with each other, the disease is extremely complex with highly variable symptoms (Lopez et al. 2011; Almási et al. 2017).
One prevalent defense strategy for disease management is resistance breeding; natural resistance genes to TSWV have been identified in tomatoes and pepper, being the most efficient Tsw gene in pepper and Sw-5 in tomato cultivars (Stevens et al. 1991; Boiteux and De Avila 1994). These resistance genes are present in some cultivars and confer high resistance to the virus, which however decreases over time because the emergence of resistance-breaking isolates (Almási et al. 2023; Lahre et al. 2023).
Therefore, given the high ability to mutate and adapt quickly to new resistant genotypes of TSWV (Qiu and Moyer 1999), it is important to perform an early detection by laboratory tests to prevent TSWV spread. As reported by the International Standard EPPO PM7/139 (EPPO 2023b), several diagnostic assays were developed for TSWV detection, some of which also validated by European “Valitest” project (Trontin et al. 2021). In detail, test plant species (Datura stramonium, Nicotiana benthamiana and N. occidentalis-P1) can be used upon mechanical inoculation to confirm TSWV infection (Roenhorst et al. 2013). To date, several serological and molecular methods are available for TSWV detection. Enzyme-linked immunosorbent assay (ELISA) (De Àvila et al. 1990), using monoclonal/polyclonal antibodies (Cho et al. 1988; Sherwood et al. 1989), and immune-gold strip kit (Yoon et al. 2014) are used for serological detection; in addition, several commercial specific antisera are available in the market (e.g. Agdia, Bioreba, etc.). Regarding molecular methods, these include hybridization with cDNA probes (Ronco et al. 1989), reverse transcription-polymerase chain reaction (RT-PCR) (Mumford et al. 19941996; Panno et al. 2012), and real-time RT-PCR (Roberts et al. 2000; Boonham et al. 2002; Mortimer-Jones et al. 2009; Debreczeni et al. 2011). Isothermal amplification methods, such as reverse transcription-dependent amplification (RT-HDA), recombinase polymerase amplification combined with lateral flow strips (RT-RPA-LFR) (Lee et al. 2021), and immunocapture reverse transcription loop-mediated isothermal amplification (IC/RT-LAMP) (Fukuta et al. 2004), have been developed for the specific TSWV detection (Wu et al. 2016). In addition, a new hyperspectral analysis model, named Outlier Removal Auxiliary Classifier Generative Adversarial Nets (OR-AC-GAN) (Wang et al. 2019) was recently reported. In this context, an early, rapid, and sensitive real time detection is an essential strategy for TSWV management.
In the last two decades, molecular detection tests based on real-time LAMP technique (Notomi et al. 2000) have provided rapid, low-cost and accurate diagnosis both in the laboratory and in the field conditions, representing an excellent tool to prevent the spread of endemic diseases and the introduction of dangerous pathogens into new geographical areas (Davino et al. 2012, 2017a; Caruso et al. 2023). Moreover, the LAMP technique, thanks to its robustness and simplicity can be used in a resource-limited context, such as in-field conditions. The robustness of the LAMP permit to analyze unprocessed samples or direct crude plant extracts to avoid total RNA/DNA extraction, as the activity of the Bst polymerase is not influenced by the presence of contaminants or inhibiting substances (Panno et al. 2020a).
In this work we developed a rapid and sensitive TSWV detection protocol based on real-time RT-LAMP. In addition, a rapid and cost-effective simplified sample homogenization procedure was evaluated allowing to use RT-LAMP directly in field.
Materials and methods
Source of viral material, RNA extraction and sample preparation
The LL-N.05 (wild type, WT—GenBank Acc. No. KP008129) and PVR (Sw-5 resistance-breaking, TBR—GenBank Acc. No. KP008134) TSWV isolates previously characterized (Debreczeni et al. 2015) kept in collection at the Instituto Valenciano de Investigaciones Agrarias—IVIA (Valencia, Spain), were used as source viral material to develop a real-time RT-LAMP assay for the specific TSWV identification. About 200 mg of fresh leaf tissue from TSWV-infected tomato plants were homogenized in a mortar with 6 ml of phosphate buffer pH 7 (0.2 M NaH2PO4, 0.2 M Na2HPO4 × 7H2O), and mechanically inoculated into five tomato and five pepper plants by rubbing the homogenate on the plants leaf surface previously sprinkled with Carborundum (320 mesh); after 48 h after mechanical inoculation, the plants were transplanted into pots with sterilized soil and placed in an insect-proof glasshouse (14/10 h photoperiod and 28/20 °C day/night target air temperature). Thirty days post-inoculations, sampling was performed from tomato and pepper plants infected with the previously reported TSWV isolates; ≈100 mg of fresh leaf tissue were homogenized in a sample extraction bag (Bioreba, Reinach, Switzerland) using the HOMEX 6 homogenizer (Bioreba, Reinach, Switzerland), with 1 mL of extraction buffer (1.3 g anhydrous sodium sulphite, 20 g polyvinylpyrrolidone MW 24–40. 000, 2 g chicken albumin grade II, 20 g Tween-20 in 1 µL distilled water, pH 7.4). Subsequently, total RNA extraction was performed using the NucleoSpin® RNA Plant kit (Macherey-Nagel GmbH & Co., Dueren, Germany), following the manufacturer’s instructions. In addition, total RNAs extracted from healthy tomato and pepper plants were used as negative controls. The total RNA concentration was measured in duplicate with the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, MA, USA); the extracted RNAs were adjusted to ≈50 ng/µL and stored at −80 °C for subsequent molecular analysis.
Primer design and TSWV detection by end-point RT-PCR
Two new primer pairs were designed for the specific detection of TSWV by end-point RT-PCR, named TSWV_SF1/TSWV_SR1 and TSWV_SF2/TSWV_SR2. In detail, the full sequences of segment S available in GenBank (JF960235, MG025804, MH367502, MF688996, KY495612, KY495611, KY495610, KY495609, KC261949, KC261952, KC261955, KC261958, KC261961, KC261967, KC261970, KC261973, KC261976, KP008129, KP008134) were aligned by using Clustal X2 program (Larkin et al. 2007) and visually analyzed to identify nucleotide regions showing a high percentage of homology at the nucleotide level. The consensus sequence was chosen as the reference sequence for the primers design, targeting the nucleotide sequence that encodes for the N (nucleocapsid protein) gene. The obtained primers were tested with the Nucleotide-BLAST algorithm (https://www.ncbi.nlm.nih.gov) to detect possible hybridization with other sequences available in GenBank, while the predicted secondary structures and hairpins were checked with Oligo Analyzer Tool (https://eu.idtdna.com/calc/analyzer). Finally, the primer sets were tested with the Vector NTI Advance 11.5 software (Invitrogen, Carlsbad, CA, USA) against the complete genomic sequences of other common viruses that affect solanaceous crops and ornamental plants (Table 1), in order to assess their affinity percentages.
A two-step end-point RT-PCR was carried out with the primer pairs obtained; in detail, the reverse-transcription (RT) was carried out in a final reaction volume of 20 µL, containing 3 µL of total RNA, 0.4 mM dNTPs, 4 µL of 5X First Strand Buffer [50 mM Tris-HCl pH 8.3, 40 mM KCl, 6 mM MgCl2] (Thermo Fisher Scientific, Waltham, MA, USA), 1 µM of reverse primer, 20 U of M-MLV reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA) and RNase-free water to reach the final volume. PCR mixture was in a final reaction volume of 25 μL, containing 2 μL of the obtained cDNA, 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 3 mM MgCl2, 0.4 mM dNTPs, 1 µM of each primer, 2 U of Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA, United States) and RNase free water to reach the final volume. The end-point RT-PCR was conducted in a MultiGene OptiMax thermal cycler (Labnet International Inc., Edison, NJ, USA) using the following cycling conditions for RT transcription: 65 °C for 10 min, 42 °C for 45 min and 95 °C for 10 min; subsequently, the PCR program for TSWV detection consisted of 1 cycle at 95 °C for 5 min, 40 cycles of 30 s at 95 °C, 40 s at the specific annealing temperature of each primer pair (56 and 50 °C for TSWV_SF1/TSWV_SR1 and TSWV_SF2/TSWV_SR2, respectively), 60 s at 72 °C and a final extension of 10 min at 72 °C. The test was conducted in duplicate in three independent assays, including a gradient temperature from 50 to 60 °C to identify the optimal annealing temperature. Healthy tomato and pepper RNAs and molecular-grade water were used as negative controls. The PCR products were electrophoresed on 1.5% (w/v) agarose gel, stained with SYBRTM Safe (Thermo Fisher Scientific, Waltham, MA, USA) and visualized by an UV light transilluminator. The obtained PCR product was sequenced in both directions using an ABI PRISM 3100 DNA sequence analyzer (Applied Biosystems, CA, United States) and the sequence was finally validated using the BLAST algorithm (https://www.ncbi.nlm.nih.gov). The primer pair that demonstrated the greatest specificity and the absence of any non-specific products was selected to conduct the subsequent experiments.
TSWV real-time RT-LAMP assay development
RT-LAMP primer design
A 500-bp nucleotide sequence elapsing the nucleotide sequence region coding for the nucleocapsid protein (N) of the consensus sequence was used for the specific LAMP primer set design. A set of six primers were selected using the Primer Explorer version 5 software (https://primerexplorer.jp/e/). In detail, the real-time RT-LAMP primer set includes two outer primers (forward and backward outer primer—F3 and B3, respectively), two inner primers (forward and backward inner primer—FIP and BIP, respectively), and two loop primers (forward and backward loop primer—LF and LB, respectively).
Moreover, TSWV real-time LAMP primer set specificity and the potential non-specific cross reactions with other viruses were assessed through in silico analysis using Nucleotide-BLAST algorithm (https://www.ncbi.nlm.nih.gov), available at the National Centre for Biotechnology Information (NCBI). In addition, to verify primer affinity, hybridization analysis was performed with the Vector NTI Advance 11.5 software (Invitrogen, Carlsbad, CA, USA), testing each primer against the full genomic sequences of other viruses (Table 1).
RT-LAMP assay optimization and specificity
The real-time RT-LAMP assay was performed in a Rotor-Gene Q 2plex HRM Platform Thermal Cycler (Qiagen, Hilden, Germany) in a final volume of 25 μL containing 0.2 μM each of TSWV-F3 and TSWV-B3, 1.6 μM each of TSWV-FIP and TSWV-BIP, 0.4 μM each of TSWV-LF and TSWV-LB, 15 μL of LAMP Isothermal Master Mix (Optigene Limited, West Sussex, United Kingdom), 3 μL of total RNA (≈50 ng/μL) previously purified from infected tomato leaves as template, and nuclease-free water to reach the final volume. In detail, the TSWV RNA previously tested by end-point RT-PCR was used for the real-time LAMP assay as positive control, while healthy tomato and pepper plant RNAs and molecular-grade water were used as negative controls. Each sample was analyzed in duplicate in three independent assays. The real-time RT-LAMP assays were performed at 65 °C for 60 min (according to manufacturer’s instructions) with fluorescence acquisition every 60 s; additional steps for melting curve calculation were carried out to record the fluorescence using the following conditions: 95 °C for 1 min, 40 °C for 1 min, 70 °C for 1 min and an increase in temperature at 0.5 °C/s up to 95 °C. The relative fluorescence unit (RFU) threshold value was used, and the threshold time (Tt) was calculated as the time at which fluorescence was equal to the threshold value, while the fluorescence data were obtained in the 6-carboxyfluorescein (FAM) channel according to the manufacturer’s instructions (excitation at 450–495 nm and detection at 510–527 nm) during the amplification.
In order to determine the specificity of the real-time RT-LAMP assay and evaluate potential non-specific reactions with other orthotospoviruses and common solanaceous-infecting viruses, a real-time RT-LAMP test was conducted using the TSWV RNA as positive control and the RNA of the viruses reported in Table 1. In each run, total RNAs from healthy tomato plants and healthy pepper plants were included; each sample was analyzed in duplicate in three independent LAMP assays. The assay was conducted using the conditions described above with the additional melting curve steps.
Sensitivity of TSWV real-time RT-LAMP assay and comparison to end point and real time RT-PCR
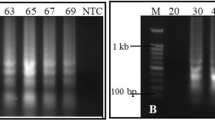
A TSWV RNA fragment carrying the target region for the RT-LAMP assay was amplified through end-point RT-PCR using the primer pair, designed in this work, that has demonstrated the highest efficiency after in silico analysis by Nucleotide-BLAST algorithm and Vector NTI Advance 11.5 software, and absence of non-specific bands. Subsequently, RT-PCR products were purified from agarose gel, using an UltraClean™ 15 DNA purification kit (MO-BIO Laboratories, Carlsbad, CA, USA). Afterwards, the purified RT-PCR product was inserted into the pGEM-T cloning vector (Promega, WI, United States) following the manufacturer’s instructions, and cloned into Escherichia coli One Shot™ Mach1™ competent cells (Invitrogen Ltd, PA, United Kingdom). The trasformants were subjected to ampicillin resistance selection, and a colony-PCR was performed to assess the fragment presence by using the primer pair previously used. Plasmid DNA purification was carried out using the NucleoSpin Plasmid DNA Purification kit (Macherey-Nagel GmbH & Co., Dueren, Germany), and the obtained plasmids were quantified twice using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, MA, USA). The plasmids were then sequenced in both directions using an ABI PRISM 3100 DNA sequence analyzer (Applied Biosystems) and the sequence was confirmed using the BLAST algorithm available at the NCBI website. Ten-fold serial dilutions were used to establish the detection limit of the RT-LAMP assay. Furthermore, end-point RT-PCR (developed in this work) and real-time RT-PCR (Roberts et al. 2000) assays were carried out, using the same serial dilutions to compare their sensitivity to real-time RT-LAMP. In detail, the ten-fold serial dilutions were obtained using the purified recombinant plasmid DNA diluted into a healthy tomato plant RNA extract, starting from a concentration of ≈50 ng/μL and diluting it up to ≈50 × 10–10 ng/μL. The number of copies was determined by calculation with the following formula:
Rapid sample preparation method suitable for the TSWV real-time RT-LAMP assay
To set up a rapid RT-LAMP assay while avoiding canonical RNA extraction, the rapid, simple and inexpensive method called “membrane spot crude extract” was used (Bertacca et al. 2022), adapting the protocol for tomato and pepper samples. A total of five tomato and five pepper plants infected with the previously reported TSWV isolates (see section “Source of viral material, RNA extraction and sample preparation”) were used. More specifically, ≈100 mg of TSWV-infected leaves, were homogenized in a sample extraction bag with 3 mL of extraction buffer. Five µL of extract was spotted on a 0.5 cm2 Hybond®-N + hybridization membrane (GE Healthcare, Chicago, IL, USA), dried at room temperature for 5 min, and placed in a 2 mL tube containing 250 µL of glycine buffer (0.1 M Glycine, 0.05 M NaCl, 1 mM EDTA). After 20 s of mixing by manual inversion, samples were heated at 95 °C for 10 min, and 3 μL of the extract were used for the real-time LAMP assay in a 25 µL final reaction volume. Healthy tomato RNA plant, healthy pepper RNA plant and molecular-grade water were used as negative controls. Each sample was analysed in duplicate in two independent real-time RT-LAMP assays, using the “membrane spot crude extract” method and the total RNA obtained with commercial kits, respectively (see section “Source of viral material, RNA extraction and sample preparation”).
Real-time RT-LAMP comparison with end-point and real time RT-PCR techniques on field samples
A total of 100 samples were collected from Trapani and Ragusa provinces (Sicily, Italy) to compare the real-time RT-LAMP results with end-point and real-time RT-PCR assays using field samples. In detail, 25 symptomatic and asymptomatic tomato plants and 25 symptomatic and asymptomatic pepper plants were tested for each province; each plant was geo-referenced with the Planthology mobile application (Davino et al. 2017b) and sampled twice to be able to carry out both in-field and laboratory analyses, using a single-hole puncher to collect seven leaf discs with a diameter of less than ~0.5 cm2 from each plant, disinfecting the tool between each sample.
Under field conditions, each sample was extracted with the “membrane spot crude extract” method and analyzed by real-time RT-LAMP assay, following the protocol described above (see section “Rapid sample preparation method suitable for the TSWV real-time RT-LAMP assay”). The in-field analysis was carried out using a ready-to-use real-time LAMP reactions, disposables, and tools as described by Caruso and co-workers (2023), adapted for TSWV detection, and performed in a bCube2 portable system (Hyris Ltd., London, United Kingdom) supplied with a 12 Volt (12 Ah) rechargeable battery.
In laboratory conditions, each sample was subjected to total RNA extraction, using the NucleoSpin® RNA Plant kit (Macherey-Nagel GmbH & Co., Dueren, Germany) (see section “Source of viral material, RNA extraction and sample preparation”), and subsequently analyzed by end-point RT-PCR, real-time RT-PCR and real-time RT-LAMP assays, using a Rotor-Gene Q 2plex HRM Platform thermal cycler (Qiagen, Hilden, Germany). Furthermore, the same samples were subjected to the “membrane spot crude extract” RNA extraction method and analyzed by real-time RT-LAMP assay in both laboratory and field conditions. Each sample was analyzed in duplicate in three independents assays. Positive and negative control RNAs were used in each diagnostic tests.
Results
Primer design and TSWV detection by end-point RT-PCR
Two primer pairs were designed for the TSWV detection through end point RT-PCR (Table 2). The results of the in silico analysis, using Nucleotide-BLAST algorithm and Vector NTI Advance 11.5 software, showed no matches with other organisms.
All primer pairs obtained were tested by end-point RT-PCR. The two tested primer pairs were both able to amplify the expected amplicons, but SF1/SR1 primer pair showed the higher specificity without non-specific bands presence. The PCR product obtained with SF1/SR1 primer pair showed the expected amplicon size of 504 bp and a percentage identity >99.9% with the TSWV sequences deposited in GenBank, indicating that the assay was specific for TSWV detection. For these reasons, the SF1/SR1 primer pair was chosen as the best candidate for TSWV detection by end-point RT-PCR.
RT-LAMP primer design
A set of six primers was designed within a 500-bp nucleotide sequence elapsing the nucleotide sequence region coding for the nucleocapsid protein (N) of segment S to set up a real-time RT-LAMP assay for rapid TSWV detection. The primer sequences and binding sites are shown in Table 3 and Fig. 1, respectively. No cross-reactions with other organisms were found when performing the in silico analysis using Nucleotide-BLAST algorithm (BLASTn tool). Furthermore, hybridization analysis carried out with the Vector NTI 11.5 program has ruled out any matches with other viruses of the Tospoviridae and Bromoviridae families reported in Table 1.
Schematic representation of reverse-transcription loop-mediated isothermal amplification (RT-LAMP) primer set (SnapGene software; available at http://www.snapgene.com) designed on the nucleocapsid coding region of TSWV Segment S. F3 and B3 are shown in orange, FIP (F1c-F2) and BIP (B1c-B2) in blue, and the two loop primers LF and LB in green. The numbers on each primer represent the binding position in the selected consensus sequence
RT-LAMP assay optimization and specificity
Using the LL-N.05 and PVR TSWV isolates, a real-time RT-LAMP assay was carried out to assess the performance of the primer set intended for TSWV detection. In Fig. 2 are reported the melting curve of the TSWV isolates showed a peak temperature of 84 °C, while the amplification curve of the isolate showed an exponential trend at ≈13 min, reaching the reaction plateau in approximately 22 min (Table 4). The negative controls did not produce any signals, even at late reaction times.
The specificity of the real-time RT-LAMP assay was evaluated to assess a potential nonspecific cross-reactions; none of the viruses reported in Table 1, used as outgroups, produced any signal, while the TSWV positive samples showed a single peak at 84 °C in the melting curve and a reaction plateau of ≈21 min. This demonstrates that the real-time RT-LAMP developed in this work is highly specific for TSWV detection.
Sensitivity of TSWV real-time RT-LAMP assay and comparison to end-point and real-time RT-PCR
The sensitivity and the efficacy of the real-time RT-LAMP assay was verified by performing a comparative experiment with the end-point and real-time RT-PCR using a ten-fold serial dilutions of a purified recombinant plasmid, starting from a concentration of ≈50 ng/µL (9.191 × 1011 copies). The real time RT-LAMP proved to be more sensitive than conventional and real-time RT-PCR assays (Table 5).
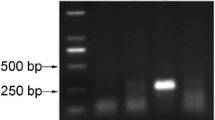
The developed assay was able to detect RNA concentrations up to ≈50 × 10–10 ng/μL (9.191 × 101 copies), while the end-point RT-PCR and the real-time RT-PCR could only detect a positive signal up to ≈50 × 10–7 ng/μL (9.191 × 104 copies) and ≈50 × 10–8 ng/μL (9.191 × 103 copies), respectively (Fig. 3).
Sensitivity of end point RT-PCR and real-time RT-LAMP assays for TSWV detection using 10-fold serial dilutions. From 101 to 10–10: 10-fold serial dilutions of a purified recombinant plasmid. Panel A: RT-PCR products electrophoresis on 2% agarose gel; M: 1kb marker; C +: positive control; NC: negative control. Panel B: Real-time RT-PCR fluorescence increasing of the 10-fold serial dilutions analyzed. Panel C: Real-time RT-LAMP fluorescence increasing of the 10-fold serial dilutions analyzed (from ≈50 × 101 ng/μL to ≈50 × 10–10 ng/μL) after 3–48 min. Panel D: Real-time RT-LAMP melting curves
These results showed that the real-time RT-LAMP assay can detect TSWV in less than 60 min (Fig. 3), even considering the lowest detectable concentration of the samples (≈50 × 10−10 ng/μL), being faster than end point RT-PCR. The melting curves displayed the same peak temperature at 84–84.5 °C, and the results of RT-LAMP reaction time plateau were calculated as the mean values obtained from the three replicates (Table 5).
Rapid sample preparation method for TSWV detection by real-time RT-LAMP assay
In order to make the TSWV real-time RT-LAMP assay faster and independent of RNA extraction standard procedures, a simple and cost-effective sample preparation method was evaluated and subsequently compared to the traditional sample preparation employing the purification commercial kit. The results showed that TSWV was detected in 16–21 min for the RNA extracted with the commercial kit, and 32–50 min for the membrane spot crude extracts (Fig. 4 and Table 6), making it a viable alternative for sample preparation. Lastly, even with this rapid procedure the negative control showed no amplification, as expected (Table 6).
Real-time RT-LAMP comparison with end-point and real time RT-PCR techniques on field samples
The real-time RT-LAMP assay developed in this study also proved to be reliable for sensitive and specific TSWV detection when applied for in-field analysis. The results of the real-time RT-LAMP performed both under laboratory conditions and in-field were comparable (Table 7).
In detail, a total of 29 out of 100 samples (all collected from symptomatic plants) resulted positive to TSWV by end-point RT-PCR, 32 out of 100 samples resulted positive by real-time RT-PCR, while 37 out of 100 samples provided positive results to TSWV infection by real-time RT-LAMP assay conducted both in-field and laboratory conditions. Specifically, a total of 13 out of 50 tomato samples resulted positive for TSWV both in-field and laboratory conditions by real-time LAMP assay. Compared to end-point RT-PCR and real-time RT-PCR, the RT-LAMP assay was able to detect TSWV infection in 3 and 2 asymptomatic tomato plants, respectively. Regarding pepper samples, a total of 24 out of 50 samples resulted positive for TSWV infection; in this case, the RT-LAMP assay was able to detect the infection in a total of 5 and 3 asymptomatic pepper plants compared to end-point RT-PCR and real-time RT-PCR, respectively.
Based on these results, the developed assay showed an increase in detecting TSWV on asymptomatic plants, in fact, the percentage of positive samples were 37, 32 and 29% with real time RT-LAMP, real-time RT-PCR and end-point RT-PCR assays, respectively. Moreover, the results were confirmed by melting curve analysis, showing the same peak temperature (84–84.5 °C) in all positive samples, including the asymptomatic ones resulted positive by RT-LAMP, concordant with the TSWV-positive controls.
Discussion
Horticultural crops are constantly threatened by different viral pathogens (Panno et al. 2021). The management of viral diseases is complex because pathogens are subject to rapid mutation and are capable of quickly adapting to different conditions, causing new outbreaks due to resistance breaking, virulence increase and the emergence of new symptoms (Acosta-Leal et al. 2011; Ferriol et al. 2013).
Losses in crop production increase every year due to the different viruses that affect plant species, resulting in a serious decrease in economic profit. The spread of new viruses and the re-emergence of existing ones in the Mediterranean basin reported in recent years emphasize the need for new effective control measures against plant pathogens (Davino et al. 2008; Panno et al. 2020b).
Thus, specific, and sensitive diagnostic and detection tools are needed to control the spread of the disease and guarantee a viable crop production. Over the years, the TSWV detection has relied mainly on ELISA and PCR-based methods; however, possible contaminations make these methods more prone to false positives, while the presence of inhibitors can cause false negatives (Ferriol et al. 2015). Among molecular diagnostic measures, the LAMP assay has been demonstrated to be highly sensitive and specific for the detection and the correct discrimination of plant pathogens, and to have a reduced sensitivity to inhibitors, making it an optimal method to use in field conditions (Panno et al. 2020a).
In this work, RT-LAMP assay has been proved to have good sensitivity, specificity, and efficiency for the detection of TSWV. A set of six primers was designed by selecting the N protein coding region as a target, and both the in silico and in vitro analysis showed good specificity for TSWV detection. The real-time RT-LAMP performed on RNA extracted from infected tomato and pepper plants proved to be more sensitive than the conventional end-point and real-time RT-PCR methods. In addition, the assay optimization revealed that reliable results can be obtained in only 60 min, which is much faster than the other RT-PCR assays. In addition, the “membrane spot crude extract” method adapted with the RT-LAMP assay developed in this work proved to be cost effective and reliable for TSWV nucleic acid extraction, compared to a conventional extraction with a commercial kit. Thus, RT-LAMP analysis of membrane spot crude extracts can be used in the field with the aid of a portable battery-powered thermal cycler, thanks to rapid sample preparation method, a ready-to-use LAMP reaction mixture and does not require skilled personnel and specific laboratory equipment. For these reasons, we propose that this technique could be used to monitor TSWV spread in laboratory and field conditions.
Data availability
Not applicable.
References
Acosta-Leal R, Duffy S, Xiong Z, Hammond R, Elena SF (2011) Advances in plant virus evolution: translating evolutionary insights into better disease management. Phytopathology 101:1136–1148. https://doi.org/10.1094/PHYTO-01-11-0017
Adkins S, Quadt R, Choi T, Ahlquist P, German TL (1995) An RNA-dependent RNA polymerase activity associated with virions of tomato spotted wilt virus, a plant- and insect- infecting Bunyavirus. Virology 207:308–311. https://doi.org/10.1006/viro.1995.1083
Almási A, Nemes K, Csömör Z, Tóbiás I, Palkovics L, Salánki K (2017) A single point mutation in Tomato spotted wilt virus NSs protein is sufficient to overcome TSW-gene-mediated resistance in pepper. J Gen Virol 98(6):1521–1525. https://doi.org/10.1099/jgv.0.000798
Almási A, Pinczés D, Tímár Z, Sáray R, Palotás G, Salánki K (2023) Identification of a new type of resistance breaking strain of tomato spotted wilt virus on tomato bearing the Sw-5b resistance gene. EurJ Plant Pathol 166:219–225. https://doi.org/10.1007/s10658-023-02656-5
Bandla MD, Campbell LR, Ullman DE, Sherwood JL (1998) Interaction of tomato spotted wilt Tospovirus (TSWV) glycoproteins with a thrips midgut protein, a potential cellular receptor for TSWV. Phytopathology 88:98–104. https://doi.org/10.1094/PHYTO.1998.88.2.98
Bellardi MG, Vicchi V (1990) TSWV: nuova insidia per la produzione agricola italiana. Informatore Fitopatologico 40(3): 17–24
Bertacca S, Caruso AG, Trippa D, Marchese A, Giovino A, Matic S, Noris E, Font MI, Alfaro A, Panno S, Davino S (2022) Development of a real-time loop-mediated isothermal amplification assay for the rapid detection of olea Europaea geminivirus. Plants 11(5):660. https://doi.org/10.3390/plants11050660
Boiteux LS, De Avila AC (1994) Inheritance of a resistance specific to tomato spotted wilt tospovirus in capsicum chinense PI 159236. Euphytica 75(1):139–142. https://doi.org/10.1007/BF00024541
Boonham N, Smith P, Walsh K, Tame J, Morris J, Spence N, Bennison J, Barker I (2002) The detection of tomato spotted wilt virus (TSWV) in individual thrips using real time fluorescent RT-PCR (TaqMan). J Virol Methods 101(1-2):37–48. https://doi.org/10.1016/S0166-0934(01)00418-9
Brittlebank CC (1919) Tomato diseases. J Dep Agric Vic 17:1348–1352
Caruso AG, Ragona A, Bertacca S, Montoya MAM, Panno S, Davino S (2023) Development of an in-field real-time LAMP assay for rapid detection of tomato leaf curl New Delhi virus. Plants 12(7):1487. https://doi.org/10.3390/plants12071487
Cho JJ, Mitchell WC, Mau LRF, Sakimura K (1987) Epidemiology of tomato spotted wilt virus on crisphead lettuce in Hawaii. Plant Dis 71:505–508. https://doi.org/10.1094/PD-71-0505
Cho JJ, Mau RFL, Hamasaki RT, Gonsalves D (1988) Detection of tomato spotted wilt virus in individual thrips by enzyme-linked immunosorbent assay. Phytopathology 78:1348–1352. https://doi.org/10.1094/Phyto-78-1348
Crescenzi A, Fanigliulo A, Viggiano A (2015) Resistance breaking tomato spotted wilt virus isolates on resistant tomato cultivars in Italy. Acta Hortic 1069:95–98. https://doi.org/10.17660/ActaHortic.2015.1069.13
Davino M, Areddia R, Durso F, Grimaldi V (1992) Indagini sul virus dell’avvizzimento maculato del pomodoro (TSWV) in Sicilia. Tecnica Agricola 42
Davino S, Accotto GP, Vaira AM, Davino M (2004) Serious viral diseases threatening protected tomato crops. Informatore Fitopatologico 54(6):35–40
Davino S, Davino M, Accotto GP (2008) A single-tube PCR assay for detecting viruses and their recombinants that cause tomato yellow leaf curl disease in the Mediterranean basin. J Virol Methods 147(1):93–98. https://doi.org/10.1016/j.jviromet.2007.08.007
Davino S, Panno S, Rangel EA, Davino M, Bellardi MG, Rubio L (2012) Population genetics of cucumber mosaic virus infecting medicinal, aromatic and ornamental plants from northern Italy. Arch Virol 157:739–745. https://doi.org/10.1007/s00705-011-1216-4
Davino S, Panno S, Iacono G, Sabatino L, DAnna F, Iapichino G, Olmos A, Scuderi G, Rubio L, Tomassoli L, Capodici G, Martinelli F, Davino M (2017a) Genetic variation and evolutionary analysis of Pepino mosaic virus in Sicily: insights into the dispersion and epidemiology. Plant Pathol 66(3):368–375. https://doi.org/10.1111/ppa.12582
Davino S, Panno S, Arrigo M, La Rocca M, Caruso AG, Bosco GL (2017b) Planthology: an application system for plant diseases management. Chem Eng Trans 58:619–624. https://doi.org/10.3303/CET1758104
Davino S, Panno S, Parrella G, Davino M, Cocuzza GEM, Rapisarda C, Caruso AG, Carpino C (2018) Viruses. In: Davino SW (ed) Tomato diseases. Viruses and Soilborne Fungi. Edizioni L’Informatore Agrario s.r.l., Verona, Italy, pp 11–137
Debreczeni DE, Ruiz-Ruiz S, Aramburu J, Lopez C, Belliure B, Galipienso L, Rubio L (2011) Detection, discrimination and absolute quantitation of Tomato spotted wilt virus isolates using real time RT-PCR with TaqMan MGB probes. J Virol Methods 176(1-2):32–37. https://doi.org/10.1016/j.jviromet.2011.05.027
Debreczeni DE, López C, Aramburu J, Darós JA, Soler S, Galipienso L, Falk BW, Rubio L (2015) Complete sequence of three different biotypes of tomato spotted wilt virus (wild type, tomato Sw-5 resistance-breaking and pepper Tsw resistance-breaking) from Spain. Arch Virol 160:2117–2123. https://doi.org/10.1007/s00705-015-2453-8
De Àvila AC, Huguenot C, Resende RDO, Kitajima EW, Goldbach RW, Peters D (1990) Serological differentiation of 20 isolates of Tomato spotted wilt virus. J Gen Virol 71:2801–2807
De Haan P, Wagemakers L, Peters D, Goldbach R (1990) The SRNA segment of tomato spotted wilt virus has an ambisense character. J Gen Virol 71:1001–1007. https://doi.org/10.1099/0022-1317-71-5-1001
De Haan P, Kormelink R, Resende RDO, van Poelwijk F, Peters D, Goldbach R (1991) Tomato spotted wilt virus L RNA encodes a putative RNA polymerase. J Gen Virol 71:2207–2216. https://doi.org/10.1099/0022-1317-72-9-2207
EPPO – European and Mediterranean Plant Protection Organization (2023a) https://www.eppo.int. Accessed 10 October 2023
EPPO – European and Mediterranean Plant Protection Organization (2023b) PM 7/139 (1) Tospoviruses. https://gd.eppo.int/download/standard/763/pm7-139-1-en.pdf
Ferriol I, Rubio L, Pérez-Panadés J, Carbonell EA, Davino S, Belliure B (2013) Transmissibility of Broad bean wilt virus 1 by aphids: influence of virus accumulation in plants, virus genotype and aphid species. Ann Appl Biol 162(1):71–79. https://doi.org/10.1111/j.1744-7348.2012.00579.x
Ferriol I, Rangel EA, Panno S, Davino S, Han CG, Olmos A, Rubio L (2015) Rapid detection and discrimination of fabaviruses by flow-through hybridisation with genus-and species-specific riboprobes. Ann Appl Biol 167(1):26–35. https://doi.org/10.1111/aab.12204
Fukuta S, Ohishi K, Yoshida K, Mizukami Y, Ishida A, Kanbe M (2004) Development of immunocapture reverse transcription loop-mediated isothermal amplification for the detection of tomato spotted wilt virus from chrysanthemum. J Virol Methods 121(1):49–55. https://doi.org/10.1016/j.jviromet.2004.05.016
Kormelink R, Storms M, Van Lent J, Peters D, Goldbach R (1994) Expression and subcellular location of the NSM protein of tomato spotted wilt virus (TSWV), a putative viral movement protein. Virology 200:56–65. https://doi.org/10.1006/viro.1994.1162
Kormelink R, Garcia ML, Goodin M, Sasaya T, Haenni AL (2011) Negative-strand RNA viruses: the plant-infecting counterparts. Virus Res 162(1-2):184–202. https://doi.org/10.1016/j.virusres.2011.09.028
Krishna-Kumar NK, Ullman DE, Cho JJ (1993) Evaluation of Lycopersicon germplasm for tomato spotted wilt virus resistance by mechanical and thrips transmission. Plant Dis 77(9):938–941
Lahre KA, Shekasteband R, Meadows I, Whitfield AE, Rotenberg D (2023) First report of resistance-breaking variants of tomato spotted wilt virus (TSWV) infecting tomatoes with the Sw-5 tospovirus-resistance gene in North Carolina. Plant Dis 107:2271. https://doi.org/10.1094/PDIS-11-22-2637-PDN
Lee HJ, Cho IS, Ju HJ, Jeong RD (2021) Rapid and visual detection of tomato spotted wilt virus using recombinase polymerase amplification combined with lateral flow strips. Mol Cell Probes 57:101727. https://doi.org/10.1016/j.mcp.2021.101727
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948
Llamas-Llamas ME, Zavaleta-Mejia E, Gonzalez-Hernandez VA, Cervantes-Diaz L, Santizo-Rincon JA, Ochoa-Martinez DL (1998) Effect of temperature on symptom expression and accumulation of tomato spotted wilt virus in different host species. Plant Pathol 47(3):341–347. https://doi.org/10.1046/j.1365-3059.1998.00249.x
Lopez C, Aramburu J, Galipienso L, Soler S, Nuez F, Rubio L (2011) Evolutionary analysis of tomato Sw-5 resistance-breaking isolates of Tomato spotted wilt virus. J Gen Virol 92(1):210–215. https://doi.org/10.1099/vir.0.026708-0
Mortimer-Jones SM, Jones MG, Jones RA, Thomson G, Dwyer GI (2009) A single tube, quantitative real-time RT-PCR assay that detects four potato viruses simultaneously. J Virol Methods 161(2):289–296. https://doi.org/10.1016/j.jviromet.2009.06.027
Moyer JW (2000) Tospoviruses. In: Hull R (ed) Encyclopedia of microbiology. Academic Press, London, pp 592–597
Mound LA (1995) The Thysanoptera vector species of tospoviruses. Acta Hortic 431:298–309. https://doi.org/10.17660/ActaHortic.1996.431.26
Moury B, Selassie KG, Marchoux G, Daubèze AM, Palloix A (1998) High temperature effects on hypersensitive resistance to tomato spotted wilt tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). EurJ Plant Pathol 104:489–498. https://doi.org/10.1023/A:1008618022144
Mumford RA, Barker I, Wood KR (1994) The detection of tomato spotted wilt virus using the polymerase chain reaction. J Virol Methods 46:303–311. https://doi.org/10.1016/0166-0934(94)90002-7
Mumford RA, Barker I, Wood KR (1996) An improved method for the detection of Tospoviruses using the polymerase chain reactions. J Virol Methods 57:109–115. https://doi.org/10.1016/0166-0934(95)01975-8
NCBI – National Center for Biotechnology Information (2023) https://www.ncbi.nlm.nih.gov/. Accessed 10 July 2023
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28(12):e63–e63. https://doi.org/10.1093/nar/28.12.e63
Oliver JE, Whitfield AE (2016) The genus Tospovirus: emerging bunyaviruses that threaten food security. Annu Rev Virol 3:101–124. https://doi.org/10.1146/annurev-virology-100114-055036
Panno S, Davino S, Rubio L, Rangel E, Davino M, García-Hernández J, Olmos A (2012) Simultaneous detection of the seven main tomato-infecting RNA viruses by two multiplex reverse transcription polymerase chain reactions. J Virol Methods 186(1-2):152–156. https://doi.org/10.1016/j.jviromet.2012.08.003
Panno S, Matić S, Tiberini A, Caruso AG, Bella P, Torta L, Stassi R, Davino S (2020a) Loop mediated isothermal amplification: principles and applications in plant virology. Plants 9(4):461. https://doi.org/10.3390/plants9040461
Panno S, Caruso AG, Barone S, Lo Bosco G, Rangel EA, Davino S (2020b) Spread of tomato brown rugose fruit virus in Sicily and evaluation of the spatiotemporal dispersion in experimental conditions. Agronomy 10(6):834. https://doi.org/10.3390/agronomy10060834
Panno S, Davino S, Caruso AG, Bertacca S, Crnogorac A, Mandić A, Noris E, Matić S (2021) A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the Mediterranean basin. Agronomy 11(11):2188. https://doi.org/10.3390/agronomy11112188
Pappu HR (2008) Tomato spotted wilt virus (Bunyaviridae). In: Brian M, MARC V (eds) Encyclopedia of virology, 3rd edn. Elsevier Ltd, Oxford, UK, pp 133–138
Qiu W, Moyer JW (1999) Tomato spotted wilt tospovirus adapts to the TSWV N gene-derived resistance by genome reassortment. Phytopathology 89(7):575–582. https://doi.org/10.1094/PHYTO.1999.89.7.575
Rampinini G (1987) Un nuovo parassita della Saintpaulia: frankliniella occidentalis. Clamer Informa 1:20–23. in Italian
Richmond KE, Chenault K, Sherwood JL, German TL (1998) Characterization of the nucleic acid binding properties of tomato spotted wilt virus nucleocapsid protein. Virology 248(6):11. https://doi.org/10.1006/viro.1998.9223
Roberts CA, Dietzgen RG, Heelan LA, Maclean DJ (2000) Real-time RT-PCR fluorescent detection of tomato spotted wilt virus. J Virol Methods 88(1):1–8. https://doi.org/10.1016/S0166-0934(00)00156-7
Roenhorst JW, Botermans M, Verhoeven JTJ (2013) Quality control in bioassays used in screening for plant viruses. EPPO Bulletin 43(2):244–249. https://doi.org/10.1111/epp.12034
Ronco AE, Dal Bó E, Ghiringhelli PD, Medrano C, Romanowski V, Sarachu AN, Grau O (1989) Cloned cDNA probes for the detection tomato spotted wilt virus. Phytopathology 79:69–74
Roselló S, Díez MJ, Nuez F (1996) Viral diseases causing the greatest economic losses to the tomato crop. I. The Tomato spotted wilt virus - a review. Sci Hortic 67(3-4):117–150. https://doi.org/10.1016/S0304-4238(96)00946-6
Saidi M, Warade SD (2008) Tomato breeding for resistance to Tomato spotted wilt virus (TSWV): an overview of conventional and molecular approaches. Czech J Genet Plant Breed 44(3):83–92. https://doi.org/10.17221/47/2008-CJGPB
Samuel G, Bald JG, Pitman HA (1930) Investigations on spotted wilt of tomatoes, Australia. Commonw Counc Sci Ind Res Bull 44
Sevik MA, Arli-Sokmen M (2012) Estimation of the effect of Tomato spotted wilt virus (TSWV) infection on some yield components of tomato. Phytoparasitica 40:87–93. https://doi.org/10.1007/s12600-011-0192-2
Sherwood JL, Sanborn MR, Keyser GC, Myer LD (1989) Use of monoclonal antibodies in detection of tomato spotted wilt virus. Phytopathology 79:61–64
Sialer MF, Parrella G, Papanice M, Vovlas C, Gallitelli D (2000) Biodiversity of viruses infecting tomato in Italy: methods for diagnosis and diversification. EPPO Bulletin 30(2):301–304. https://doi.org/10.1111/j.1365-2338.2000.tb00900.x
Smith IM, McNamara DG, Scott PR, Harris KM (1992) Frankliniella occidentalis. In: CAB/EPPO (ed) Quarantine Pests for Europe. University Press, Cambridge, pp 145
Stevens MR, Scott SJ, Gergerich RC (1991) Inheritance of a gene for resistance to tomato spotted wilt virus (TSWV) from Lycopersicon peruvianum Mill. Euphytica 59(1):9–17. https://doi.org/10.1007/BF00025356
Takeda A, Sugiyama K, Nagano H, Mori M, Kaido M, Mise K, Tsuda S, Okuno T (2002) Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Lett 532(1-2):75–79. https://doi.org/10.1016/S0014-5793(02)03632-3
Triolo E, Materazzi A, Resta E (1991) II virus dell’avvizzimento maculato del pomodoro (TSWV) su crisantemo in Italia. Notiziario Sulle Malattie Delle Piante 112:76–88
Trontin C, Agstner B, Altenbach D, Anthoine G, Bagińska H, Brittain I, Vučurović A (2021) VALITEST: validation of diagnostic tests to support plant health. Eppo Bulletin 51(1):198–206. https://doi.org/10.1111/epp.12740
Ullman DE, German TL, Sherwood JL, Westcot DM, Cantone FA (1993) Tospovirus replication in insect vector cells: immuno-cytochemical evidence that the nonstructural protein encoded by the S RNA of tomato spotted wilt tospovirus is present in thrips vector cells. Phytopathology 83:456–463
Ullman DE, Meideros R, Campbell LR, Whitfield AE, Sherwood JL (2002) Thrips as vectors of tospoviruses. In: Plumb R (ed) Advances in botanical research. Elsevier Science Ltd, pp 113–140
Wang D, Vinson R, Holmes M, Seibel G, Bechar A, Nof S, Tao Y (2019) Early detection of tomato spotted wilt virus by hyperspectral imaging and outlier removal auxiliary classifier generative adversarial nets (OR-AC-GAN). Sci Rep 9(1):1–14. https://doi.org/10.1038/s41598-019-40066-y
Wang H, Wu X, Huang X, Wei S, Lu Z, Ye J (2022) Seed transmission of tomato spotted wilt orthotospovirus in peppers. Viruses 14(9):1873. https://doi.org/10.3390/v14091873
Wijkamp I, van Lent J, Goldbach R, Peters D (1993) Multiplication of tomato spotted wilt virus in its vector, Frankliniella occidentalis. J Gen Virol 74:341–349. https://doi.org/10.1099/0022-1317-74-3-341
Wu X, Chen C, Xiao X, Deng MJ (2016) Development of reverse transcription thermostable helicase-dependent DNA amplification for the detection of tomato spotted wilt virus. J AOAC Int 99(6):1596–1599. https://doi.org/10.5740/jaoacint.16-0132
Yoon JY, Choi GS, Cho IS, Choi SK (2014) Development of rapid immune-gold strip kit for on-site diagnosis of tomato spotted wilt virus. Res Plant Dis 20(1):15–20. https://doi.org/10.5423/RPD.2014.20.1.015
Acknowledgements
The authors thank the “SiciliAn MicronanOTecH Research and Innovation CEnter SAMOTHRACE (MUR, PNRR-M4C2, ECS_00000022), spoke 3–University of Palermo S2-COMMs-Micro and Nanotechnologies for Smart and Sustainable Communities”.
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement. This research was funded by “MUR (Ministero dell’Università e della Ricerca), PNRR-M4C2, ECS_00000022”; MCI (Ministerio de Ciencia e Innovación), PID2021-125787OR-C31 and IVIA 52202J, co-financed by FEDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Informed consent was obtained from each individual participants included in the study.
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caruso, A., Ragona, A., Agrò, G. et al. Rapid detection of tomato spotted wilt virus by real-time RT-LAMP and in-field application. J Plant Pathol 106, 697–712 (2024). https://doi.org/10.1007/s42161-024-01613-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-024-01613-3