Abstract
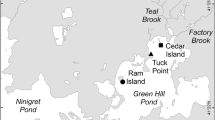
The influence of oysters on nitrogen (N) cycling has received increased research attention. Previous work focused on fluxes of N solutes and gases, but the effects on microbes responsible for N transformations are unknown. In May 2010, we deployed eastern oysters (Crassostrea virginica) in mesh cages above sand-filled boxes at four sites across a nutrient gradient in Jamaica Bay, New York City. In fall and winter, we used quantitative PCR to measure abundance of 16S rRNA and nitrite reductase genes for denitrification (nirS and nirK) and dissimilatory nitrate reduction to ammonium (nrfA) in sediment. We measured water column nutrients and chlorophyll a, sediment C:N and organic matter (OM), exchangeable ammonium (NH4 +), ammonification, nitrification, and denitrification potential (DNP). Oysters did not affect gene abundance in fall, when we predicted that their influence would be strongest, or in winter. However, gene abundance was significantly different among sites and seasons. Factors which explained 16S rRNA, nirS, and nirK gene abundance included sediment OM, water column N, and chlorophyll a, similar to previous research. Abundance of nrfA was lower than that of nir genes and positively related to sediment C:N, suggesting OM lability may drive the balance between nir and nrfA. Finally, nirS and nirK abundance was unrelated to DNP, which is consistent with variable results from the literature. More studies that combine molecular techniques with N transformation rates in the context of oyster reefs are needed. Results will advance models which predict the ecosystem effects of reef conservation and restoration under variable environmental conditions.



Similar content being viewed by others
References
Abell, G., D. Ross, J. Keane, J.M. Oakes, B.D. Eyre, S. Robert, and J. Volkman. 2013. Nitrifying and denitrifying microbial communities and their relationship to nutrient fluxes and sediment geochemistry in the Derwent Estuary, Tasmania. Aquatic Microbial Ecology 70: 63–75.
Attard, E., S. Recous, A. Chabbi, C. De Berranger, N. Guillaumaud, J. Labreuche, L. Philippot, B. Schmid, and X. Le Roux. 2011. Soil environmental conditions rather than denitrifier abundance and diversity drive potential denitrification after changes in land uses. Global Change Biology 17: 1975–1989.
Azandégbé, A., F. Poly, F. Andrieux-Loyer, R. Kérouel, X. Philippon, and J.L. Nicolas. 2012. Influence of oyster culture on biogeochemistry and bacterial community structure at the sediment–water interface. FEMS Microbiology Ecology 82: 102–117.
Banerjee, S., and S.D. Siciliano. 2012. Factors driving potential ammonia oxidation in Canadian arctic ecosystems: does spatial scale matter? Applied and Environmental Microbiology 78: 346–353.
Barrett, M., M.M. Jahangir, C. Lee, C.J. Smith, N. Bhreathnach, G. Collins, K.G. Richards, and V. O’Flaherty. 2013. Abundance of denitrification genes under different peizometer depths in four Irish agricultural groundwater sites. Environmental Science and Pollution Research 20: 6646–6657.
Baudoin, E., L. Philippot, D. Chèneby, L. Chapuis-Lardy, N. Fromin, D. Bru, B. Rabary, and A. Brauman. 2009. Direct seeding mulch-based cropping increases both the activity and the abundance of denitrifier communities in a tropical soil. Soil Biology and Biochemistry 41: 1703–1709.
Baxter, A., L. Johnson, T. Royer, and L. Leff. 2013. Spatial differences in denitrification and bacterial community structure of streams: relationships with environmental conditions. Aquatic Sciences 75: 275–284.
Beck, M.W., R.D. Brumbaugh, L. Airoldi, A. Carranza, L.D. Coen, C. Crawford, O. Defeo, G.J. Edgar, B. Hancock, M.C. Kay, H.S. Lenihan, M.W. Luckenbach, C.L. Toropova, G. Zhang, and X. Guo. 2011. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61: 107–116.
Beman, J.M. 2014. Activity, abundance, and diversity of nitrifying archaea and denitrifying bacteria in sediments of a subtropical estuary, 1343–1352. Bahía del Tóbari, Mexico: Estuaries and Coasts.
Benotti, M. J., M. Abbene, and S. A. Terracciano. 2007. Nitrogen loading in Jamaica Bay, Long Island, New York: predevelopment to 2005: U.S. Geological Survey Scientific Investigations Report 2007–5051. 17 p., online only.
Black, F. R. 1981. Jamaica Bay: A History, ed. U. S. National Parks Service, 104. Washington, D.C.
Bowen, J.L., A.R. Babbin, P.J. Kearns, and B.B. Ward. 2014. Connecting the dots: linking nitrogen cycle gene expression to nitrogen fluxes in marine sediment mesocosms. Frontiers in Microbiology 5: 429.
Braker, G., A. Fesefeldt, and K.-P. Witzel. 1998. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Applied and Environmental Microbiology 64: 3769–3775.
Bulow, S.E., C.A. Francis, G.A. Jackson, and B.B. Ward. 2008. Sediment denitrifier community composition and nirS gene expression investigated with functional gene microarrays. Environmental Microbiology 10: 3057–3069.
Burgin, A.J., and S.K. Hamilton. 2007. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5: 89–96.
Cerco, C.F., and M.R. Noel. 2007. Can oyster restoration reverse cultural eutrophication in Chesapeake Bay? Estuaries and Coasts 30: 331–343.
Correa-Galeote, D., D.E. Marco, G. Tortosa, D. Bru, L. Philippot, and E.J. Bedmar. 2013. Spatial distribution of N‐cycling microbial communities showed complex patterns in constructed wetland sediments. FEMS Microbiology Ecology 83: 340–351.
Coyne, M.S., A. Arunakumari, B.A. Averill, and J.M. Tiedje. 1989. Immunological identification and distribution of dissimilatory heme cd1 and nonheme copper nitrite reductases in denitrifying bacteria. Applied and Environmental Microbiology 55: 2924–2931.
Dandie, C.E., D.L. Burton, B.J. Zebarth, S.L. Henderson, J.T. Trevors, and C. Goyer. 2008. Changes in bacterial denitrifier community abundance over time in an agricultural field and their relationship with denitrification activity. Applied and Environmental Microbiology 74: 5997–6005.
Desnues, C., V.D. Michotey, A. Wieland, C. Zhizang, A. Fourçans, R. Duran, and P.C. Bonin. 2007. Seasonal and diel distributions of denitrifying and bacterial communities in a hypersaline microbial mat (Camargue, France). Water Research 41: 3407–3419.
Djigal, D., E. Baudoin, L. Philippot, A. Brauman, and C. Villenave. 2010. Shifts in size, genetic structure and activity of the soil denitrifier community by nematode grazing. European Journal of Soil Biology 46: 112–118.
Dong, L.F., C.J. Smith, S. Papaspyrou, A. Stott, A.M. Osborn, and D.B. Nedwell. 2009. Changes in benthic denitrification, nitrate ammonification, and anammox process rates and nitrate and nitrite reductase gene abundances along an estuarine nutrient gradient (the Colne Estuary, United Kingdom). Applied and Environmental Microbiology 75: 3171–3179.
Dong, L.F., M.N. Sobey, C.J. Smith, I. Rusmana, W. Phillips, A. Stott, A.M. Osborn, and D.B. Nedwell. 2011. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnology and Oceanography 56: 279–291.
Dunn, R.J.K., D. Robertson, P.R. Teasdale, N.J. Waltham, and D.T. Welsh. 2013. Benthic metabolism and nitrogen dynamics in an urbanised tidal creek: domination of DNRA over denitrification as a nitrate reduction pathway. Estuarine, Coastal and Shelf Science 131: 271–281.
Einsle, O., and P.M.H. Kroneck. 2004. Structural basis of denitrification. Biological Chemistry 385: 875–883.
Enwall, K., I.N. Throback, M. Stenberg, M. Soderstrom, and S. Hallin. 2010. Soil resources influence spatial patterns of denitrifying communities at scales compatible with land management. Applied and Environmental Microbiology 76: 2243–2250.
Eyre, B.D., and A.J.P. Ferguson. 2005. Benthic metabolism and nitrogen cycling in a subtropical east Australian Estuary (Brunswick): temporal variability and controlling factors. Limnology and Oceanography 50: 81–96.
Fazzolari, É., B. Nicolardot, and J.C. Germon. 1998. Simultaneous effects of increasing levels of glucose and oxygen partial pressures on denitrification and dissimilatory nitrate reduction to ammonium in repacked soil cores. European Journal of Soil Biology 34: 47–52.
Franz, D.R. 1982. An historical perspective on mollusks in Lower New York Harbor, with emphasis on oysters. In Ecological stress and the New York bight: science and management, ed. G.F. Meyer. Columbia SC: Estuarine Research Federation.
Freeman, W.M., S.J. Walker, and K.E. Vran. 1999. Quantitative RT-PCR: pitfalls and potential. BioTechniques 26: 112–125.
Fulweiler, R.W., S.M. Brown, S.W. Nixon, and B.D. Jenkins. 2013. Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Marine Ecology Progress Series 482: 57–68.
Galstoff, P. S. 1964. The American oyster, Crassotrea virginica, ed. F. B. N. 64, 480. Washington, DC: United States Government Printing Office.
Gardner, W.S., and M.J. McCarthy. 2009. Nitrogen dynamics at the sediment-water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 95: 185–198.
Giacomucci, L., K. Purdy, E. Zanardini, A. Polo, and F. Cappitelli. 2011. A new non-degenerate primer pair for the specific detection of the nitrite reductase gene nrfA in the genus Desulfovibrio. Journal of Molecular Microbiology and Biotechnology 22: 345–351.
Giblin, A.E., C.R. Tobias, B. Song, N. Weston, G.T. Banta, and V.H. Rivera-Monroy. 2013. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26: 124–131.
Grabowski, J.H., and C.H. Peterson. 2007. Restoring oyster reefs to recover ecosystem services. In Ecosystem engineers: plants to protists, ed. K. Cuddington, J.E. Byers, W.G. Wilson, and A. Hastings, 281–298. Amsterdam: Academic.
Grabowski, J.H., R.D. Brumbaugh, R.F. Conrad, A.G. Keeler, J.J. Opaluch, C.H. Peterson, M.F. Piehler, S.P. Powers, and A.R. Smyth. 2012. Economic valuation of ecosystem services provided by oyster reefs. Bioscience 62: 900–909.
Green, S.J., O. Prakash, T.M. Gihring, D.M. Akob, P. Jasrotia, P.M. Jardine, D.B. Watson, S.D. Brown, A.V. Palumbo, and J.E. Kostka. 2010. Denitrifying bacteria isolated from terrestrial subsurface sediments exposed to mixed-waste contamination. Applied and Environmental Microbiology 76: 3244–3254.
Groffman, P.M., M.A. Altabet, J.K. Bohlke, K. Butterbach-Bahl, M.B. David, M.K. Firestone, A.E. Giblin, T.M. Kana, L.P. Nielsen, and M.A. Voytek. 2006. Methods for measuring denitrification: diverse approaches to a difficult problem. Ecological Applications 16: 2091–2122.
Hallin, S., C.M. Jones, M. Schloter, and L. Philippot. 2009. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. The ISME Journal 3: 597–605.
Herbert, R.A. 1999. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiology Reviews 23: 563–590.
Higgins, C.B., C. Tobias, M.F. Piehler, A.R. Smyth, R.F. Dame, K. Stephenson, and B.L. Brown. 2013. Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Marine Ecology Progress Series 473: 7–27.
Hoellein, T.J., and C.B. Zarnoch. 2014. Effect of eastern oysters (Crassostrea virginica) on sediment carbon and nitrogen dynamics in an urban estuary. Ecological Applications 24: 271–286.
Hoellein, T.J., C.B. Zarnoch, and R. Grizzle. 2015. Eastern oyster (Crassostrea virginica) filtration, biodeposition, and sediment nitrogen cycling at two oyster reefs with contrasting water quality in Great Bay Estuary (New Hampshire, USA). Biogeochemistry 122: 113–129.
Huang, S., C. Chen, X. Yang, Q. Wu, and R. Zhang. 2011. Distribution of typical denitrifying functional genes and diversity of the nirS-encoding bacterial community related to environmental characteristics of river sediments. Biogeosciences 8: 3041–3051.
Kandeler, E., K. Deiglmayr, D. Tscherko, D. Bru, and L. Philippot. 2006. Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Applied and Environmental Microbiology 72: 5957–5962.
Kellogg, L.M., J.C. Cornwell, M.S. Owens, and K.T. Paynter. 2013. Denitrification and nutrient assimilation on a restored oyster reef. Marine Ecology Progress Series 480: 1–19.
Kellogg, M.L., A.R. Smyth, M.W. Luckenbach, R.H. Carmichael, B.L. Brown, J.C. Cornwell, M.F. Piehler, M.S. Owens, D.J. Dalrymple, and C.B. Higgins. 2014. Use of oysters to mitigate eutrophication in coastal waters. Estuarine, Coastal and Shelf Science 151: 156–168.
Kembel, S.W., M. Wu, J.A. Eisen, and J.L. Green. 2012. Incorporating 16S gene copy number information improves estimates of microbial diversity and abundance. Plos Computational Biology 8: e1002743.
Knapp, C.W., W.K. Dodds, K.C. Wilson, J.M. O’Brien, and D.W. Graham. 2009. Spatial heterogeneity of denitrification genes in a highly homogenous urban stream. Environmental Science & Technology 43: 4273–4279.
Lam, P., G. Lavik, M.M. Jensen, J. van de Vossenberg, M. Schmid, D. Woebken, D. Gutiérrez, R. Amann, M.S. Jetten, and M.M. Kuypers. 2009. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proceedings of the National Academy of Sciences 106: 4752–4757.
Langdon, C.J., and R.I.E. Newell. 1996. Digestion and nutrition in larvae and adults. In The eastern oyster Crassostrea virginica, ed. V.S. Kennedy, R.I.E. Newell, and A.F. Eble, 231–269. College Park: Maryland Sea Grant.
Levinton, J., M. Doall, D. Ralston, A. Starke, and B. Allam. 2011. Climate change, precipitation and impacts on an estuarine refuge from disease. Plos One 6: e18849.
Levy-Booth, D.J., and R.S. Winder. 2010. Quantification of nitrogen reductase and nitrite reductase genes in soil of thinned and clear-cut Douglas-fir stands by using real-time PCR. Applied and Environmental Microbiology 76: 7116–7125.
López-Gutiérrez, J.C., S. Henry, S. Hallet, F. Martin-Laurent, G. Catroux, and L. Philippot. 2004. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. Journal of Microbiological Methods 57: 399–407.
Morales, S.E., T. Cosart, and W.E. Holben. 2010. Bacterial gene abundances as indicators of greenhouse gas emission in soils. The ISME Journal 4: 799–808.
Morrissey, E.M., A.S. Jenkins, B.L. Brown, and R.B. Franklin. 2013. Resource availability effects on nitrate-reducing microbial communities in a freshwater wetland. Wetlands 33: 301–310.
Mosier, A.C., and C.A. Francis. 2010. Denitrifier abundance and activity across the San Francisco Bay estuary. Environmental Microbiology Reports 2: 667–676.
Muyzer, G., S. Hottentrager, A. Teske, and C. Wawer. 1996. Denaturing gradient gel electrophoresis of PCR-amplified 16S rDNA. A new molecular approach to analyze the genetic diversity of mixed microbial communities. In Molecular microbial ecology manual, ed. A.D.I. Akkermans, J.D. Van Elsas, and F.J. De Bruijn, 3.4.4.1–3.4.4.22. Dordrecht: Kluwer.
Newell, R.I.E., and C.J. Langdon. 1996. Mechanisms and physiology of larval and adult feeding. In The eastern oyster Crassostrea virginica, ed. K.V.S.R.I.E. Newell and A.F. Eble, 185–229. College Park: Maryland Sea Grant.
Newell, R.I.E., J.C. Cornwell, and M.S. Owens. 2002. Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: a laboratory study. Limnology and Oceanography 47: 1367–1379.
Newell, R.I.E., T.R. Fisher, R.R. Holyoke, and J.C. Cornwell. 2005. Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. In The comparative roles of suspension feeders in ecosystems, ed. R. Dame and S. Olenin, 93–120. Netherlands: NATO Science Series: IV - Earth and Environmental Sciences. Springer.
Nizzoli, D., D.T. Welsh, E.A. Fano, and P. Viaroli. 2006. Impact of clam and mussel farming on benthic metabolism and nitrogen cycling, with emphasis on nitrate reduction pathways. Marine Ecology Progress Series 315: 151–165.
Nolan, T., R.E. Hands, and S.A. Bustin. 2006. Quantification of mRNA using real-time RT-PCR. Nature Protocols 1: 1559–1582.
Peng, X., E. Yando, E. Hildebrand, C. Dwyer, A. Kearney, A. Waciega, I. Valiela, and A. Bernhard. 2013. Differential responses of ammonia-oxidizing archaea and bacteria to long-term fertilization in a New England salt marsh. Frontiers in Microbiology 3
Petersen, D.G., S.J. Blazewicz, M. Firestone, D.J. Herman, M. Turetsky, and M. Waldrop. 2012. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environmental Microbiology 14: 993–1008.
Piehler, M. F., and A. R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2: art12.
Priemé, A., G. Braker, and J.M. Tiedje. 2002. Diversity of nitrite reductase (nirK and nirS) gene fragments in forested upland and wetland soils. Applied and Environmental Microbiology 68: 1893–1900.
R Development Core Team. 2009. R: a language and environment for statistical computing: R Foundation for Statistical Computing. http://www.R-project.org.
Seitzinger, S., J.A. Harrison, J.K. Bohlke, A.F. Bouwman, R. Lowrance, B. Peterson, C. Tobias, and G.V. Drecht. 2006. Denitrification across landscapes and waterscapes: a synthesis. Ecological Applications 16: 2064–2090.
Smith, M.S., and J.M. Tiedje. 1979. Phases of denitrification following oxygen depletion in soil. Soil Biology and Biochemistry 11: 261–267.
Smith, C.J., D.B. Nedwell, L.F. Dong, and A.M. Osborn. 2007. Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Applied and Environmental Microbiology 73: 3612–3622.
Smyth, A.R., N.R. Geraldi, and M.F. Piehler. 2013a. Oyster-mediated benthic-pelagic coupling modifies nitrogen pools and processes. Marine Ecology Progress Series 493: 23–30.
Smyth, A.R., S.P. Thompson, K.N. Siporin, W.S. Gardner, M.J. McCarthy, and M.F. Piehler. 2013b. Assessing nitrogen dynamics throughout an estuarine landscape. Estuaries and Coasts 36: 44–55.
Song, K., S.-H. Lee, W.J. Mitsch, and H. Kang. 2010. Different responses of denitrification rates and denitrifying bacterial communities to hydrologic pulsing in created wetlands. Soil Biology and Biochemistry 42: 1721–1727.
Song, B., J. A. Lisa, and C. R. Tobias. 2014. Linking DNRA community structure and activity in a shallow lagoonal estuarine system. Frontiers in Microbiology 5
Takeuchi, J. 2006. Habitat segregation of a functional gene encoding nitrate ammonification in estuarine sediments. Geomicrobiology Journal 23: 75–87.
Throback, I.N., K. Enwall, A. Jarvis, and S. Hallin. 2004. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiology Ecology 49: 401–417.
Tiedje, J. M., S. Simkins, and P. M. Groffman. 1989. Perspectives on measurement of denitrification in the field including recommended protocols for acetylene based methods. In Ecology of arable land, ed. M. Clarholm and L. Bergstrom, 217–240.
Wallenstein, M.D., D.D. Myrold, M. Firestone, and M. Voytek. 2006. Environmental controls on denitrifying communities and denitrification rates: insights from molecular methods. Ecological Applications 16: 2143–2152.
Wang, H., and R.P. Gunsalus. 2000. The nrfA and nirB nitrite reductase operons in Escherichia coli are expressed differently in response to nitrate than to nitrite. Journal of Bacteriology 182: 5813–5822.
Warneke, S., L.A. Schipper, M.G. Matiasek, K.M. Scow, S. Cameron, D.A. Bruesewitz, and I.R. McDonald. 2011. Nitrate removal, communities of denitrifiers and adverse effects in different carbon substrates for use in denitrification beds. Water Research 45: 5463–5475.
Wigand, C., C.T. Roman, E. Davey, M. Stolt, R. Johnson, A. Hanson, E.B. Watson, S.B. Moran, D.R. Cahoon, J.C. Lynch, and P. Rafferty. 2014. Below the disappearing marshes of an urban estuary: historic nitrogen trends and soil structure. Ecological Applications 24: 633–649.
Zumft, W.G. 1997. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61: 533–616.
Acknowledgments
Funding was provided by the National Science Foundation Awards DEB-0918952 and MRI-0959876, PSC-CUNY Research Awards, Eugene Lang Junior Faculty Fellowship, and Loyola University Chicago Provost Fellowship and Biology Summer Research Fellowship. We thank graduate students Allison Mass Fitzgerald, Michael Hassett, Corrina Singleman, and Kayla Turek and undergraduate assistants Elizabeth Humphrey, Narendra Paramanand, Swathi Mummini, Doris Law, Angeline David, Simon Morgan, Steven Polaskey, Gena Isreal, Owen McKenna, Adam Pink, Yana Zak, Nouran Aly, Vitaly Zaharov, Hanen Yan, Kevin Kucher, Jemi Jacob, and Sanne Lynham for field and laboratory assistance. We thank Dr. Stefan Kanzok for qPCR support. Institutional support was provided by Baruch College, Loyola University Chicago, Gateway National Recreation Area, Jamaica Bay Wildlife Refuge Visitor Center, NYS Department of Environmental Conservation, and the Jamaica Bay Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bongkeun Song
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Mean (±SE) relative abundance of functional gene copy numbers per abundance of 16S rRNA gene copies from Floyd Bennett Field (FB), Wildlife Refuge (WR), Mott’s Basin (MB), and Spring Creek (SC) for nirS in A) fall and B) winter, nirK in C) fall and D) winter, nrfA in E) fall and F) winter (GIF 50 kb)
Rights and permissions
About this article
Cite this article
Lindemann, S., Zarnoch, C.B., Castignetti, D. et al. Effect of Eastern Oysters (Crassostrea virginica) and Seasonality on Nitrite Reductase Gene Abundance (nirS, nirK, nrfA) in an Urban Estuary. Estuaries and Coasts 39, 218–232 (2016). https://doi.org/10.1007/s12237-015-9989-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-9989-4