Abstract
Potatoes are highly consumed food around the world, usually following processing of some kind. Apart from its noteworthy presence in diets, potato starch has a multitude of industrial applications as a food additive and recently in novel domains such as nanotechnology and bioengineering. This review examines the microscopic and spectroscopic methods of characterizing potato starch and compares the different properties. The microscopic techniques such as optical microscopy and Scanning Electron Microscopy (SEM) allow observation of structural elements of potato starch. Differential Scanning Calorimetry (DSC) delves into the thermal behavior of starch in presence of water, while Fourier Transform Infrared (FTIR) spectroscopy and X-Ray Diffraction (XRD) analyze the behavior of various chemical bonds and crystallinity of starch. These characterizations are important from a dietary point of view for patients requiring a low-glycemic diet, as well as in facilitating research into a wider array of industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Las papas son un alimento de alto consumo al rededor del mundo, generalmente después de seguir procesamiento de algún tipo. Además de su notable presencia en dietas, el almidón de la papa tiene una multitud de aplicaciones industriales como aditivo de los alimentos y recientemente en dominios novedosos tales como la nanotecnología y la bioingeniería. Esta revisión examina los métodos microscópicos y espectroscópicos de la caracterización del almidón de la papa y compara las diferentes propiedades. Las técnicas microscópicas tales como la microscopía óptica y el microscopio electrónico de barrido (SEM) permiten la observación de los elementos estructurales del almidón de la papa. El barrido diferencial calorimétrico (DSC) profundiza en el comportamiento térmico del almidón en presencia de agua, mientras que la espectroscopía de transformación infrarroja Fourier (FTIR) y la difracción por rayos X (XRD), analizan el comportamiento de varios enlaces químicos y la cristalinidad del almidón. Estas caracterizaciones son importantes desde un punto de vista de la dieta para pacientes que requieran una dieta baja en azúcar, así como en investigación facilglicemicitante dentro de un amplio arreglo de aplicaciones industriales.
Introduction
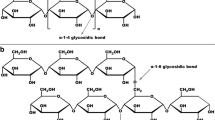
Found as one of the most abundant components of daily diets, starch is a widely consumed carbohydrate found in a plethora of food sources, such as grains, tubers and vegetables. Among these, potatoes are the most consumed tuber and third-largest food crop in the world (Wang and Copeland 2013). Starch is a polysaccharide consisting of multiple glucose units held together by glycosidic bonds (Van der Maarel et al. 2002) and is synthesized in the amyloplasts of plant cells in granular form. There are two main polymers of glucose units that make up starch; amylose is a straight-chain molecule that consists of α-1,4 glycosidic bonds while amylopectin is a branched-chain molecule that consists of both α-1,4 and α-1,6 glycosidic bonds, where the latter of the two bonds is found at regions of branching of a glucose molecule away from the main chain (Kowsik and Mazumder 2018).Starch, being one of the most highly consumed nutrient subgroups, plays a greater role in our metabolism than previously understood. Studies have theorised starch as being a driving force of the evolution of humans, with evidence suggesting that the prevalence of starch in the diets of early hominids enabled the evolution of their brains (Hardy et al. 2015). This is based on genetic studies of hominid amylase genes based on archaeological evidence, which explains the optimization of our physiology with our diets, and the link between consumption of carbohydrate-containing foods and the evolution of our metabolism, which paved the way for better-developed brains and digestive systems. In humans, enzyme-driven starch digestion starts in the mouth by the action of salivary α- amylase. After a series of mechanical breakdowns and processing in the stomach, the action of pancreatic α-amylase commences. These α-amylases specifically target and cleave α-1, 4 glycosidic linkages. This hydrolysis converts huge polysaccharide into short linear oligomers and branched glucans. Studies carried out in porcine pancreatic α-amylase have shown that it is mainly an endo-amylase, which means that it hydrolyses the glycosidic linkages randomly at the non-terminal ends of amylase chain. (Brownlee et al. 2018; Drummond et al. 1971). Apart from the two α-amylases, the brush border of the intestinal epithelium is associated with several glucosidases such as lactase, maltase, isomaltase, sucrase and dextrinases, which carry out the final digestion of starch and release glucose into the blood stream (Brownlee et al. 2018).
Starch extracted from naturally occurring food sources is widely used as an additive in numerous food-related industries as a stabilizer, gelling agent, thickener and as a substitute for fats in foods (De Olivera et al. 2018). Recent years have seen a shift in the use of potatoes in developed countries, where the commercial uses have outweighed the consumption of fresh potatoes as food. Starch biopolymers have also been derived for pharmaceutical purposes as nano-drug delivery systems and cell and tissue bioengineering applications. The pharmaceutical ingredient is incorporated with the starch particles, which are often combined with other biopolymers to create a stable, non-toxic drug delivery system capable of targeted drug delivery. The main advantages of using starch are centered on its low cost and relative purity when compared to other biopolymers, thus avoiding the need to employ purification procedures before usage (Rodrigues and Emeje 2012). Starch has also been extensively used in numerous bioplastic formulations due to its ability to form a film when gelatinized, as well as an easy formulation with other polymers to create a more stable structure (Saliu et al. 2019). The biodegradability of starch-based plastics has made them desirable as an eco-friendly alternative.
Potato Starch in Processed Foods and their Health Impact
The fourth most vital vegetable crop in the world is Potato. Due to the widespread consumption of carbohydrate-based foods, starch has become a dietary factor that supplies a vast majority of the world’s population with energy utilized for metabolism (Dupuis and Liu 2019). Starches are widely used in the food industry for the manufacture of starch-based foods. Certain properties of starch-based foods such as gelation, retrogradation, and rheological properties are affected by the types and concentration of starch used (Acquarone and Rao 2003). The effect of starch on the health of individuals is linked to the nature of the starch molecules, which in turn affect the digestibility of the starch and consequently the glycemic levels in the blood. The digestive property of potato starch is impacted by the food matrix. Starch can be classified broadly based on their digestibility into Rapid-Digestible Starch (RDS), Slow-Digesting Starch (SDS), and Resistant Starch (RS), as seen in Table 1. Resistant starch can be further classified into four categories based upon the reason for its resistance to digestion (Table 2) (Englyst et al. 1996). The RS content in processed food is negatively correlated with the other types of carbohydrates (simple sugars, gums, and fibers) present in it. The presence of proteins, oils, and other salts also negatively correlates with the RS content of the food (Escarpa et al. 1997). The formation of RS can be hindered by the addition of proteins to the potato starch. This can be due to the possible reduction of an enzymatic attack on the resulting potato starch-protein complex. Digestibility of starch was reduced when the ingredients like Xanthum gum, guar gum, pectin, and glucomannan were added during the processing of starch based-food products. Sasaki et al. 2015 found the increased RS content in potato starch gels on the in vitro addition of these ingredients at 5,10 or 15% (5% Xanthum gum were an exception) respectively. The processed potatoes are utilized as table stock, frozen French fries, potato chips, shoestrings, and dehydrated items (Miranda and Aguilera 2006). The processing of food items can greatly influence the RS and SDS content in it (Bao 2017).
The quality of potato products is highly influenced by processing and storage conditions. For instance, cold storage conditions for potatoes have shown to be linked with the accumulation of sugars, primarily glucose, sucrose and fructose by the process called cold sweetening (Sowokinos 2001). This cold sweetening is the result of starch phosphorylase activity at the initial stage; the eventual action of certain amylolytic enzymes results in the release and accumulation of reducing sugars in the potatoes and other tubers (Morrell and Ap Rees 1986). Conversely, during high temperature processing of potato food products (above 100 °C), the Maillard reaction takes place, whereby interactions occur between sugars, asparagine and the specific amino acids found in the food. This reaction is responsible for the undesirable dark-colored products such as acrylamide, and formation of melanoidins which have a bitter taste. However, the Maillard reaction is necessary to some extent for the formation of color and flavor in manufactured fried potatoes. The susceptibility to the formation of acrylamide is due to the presence of abundant free asparagine in potatoes. The color of the fried potato products also depends on the superficial reducing sugars present in the potatoes, the frying temperature, and time period (Zyzak et al. 2003; Marquez and Anon 1986).
The quality of starch used in the manufacture of processed food plays a vital role in the health of an individual. Excess consumption of carbs rich food may lead to diabetes, cardiovascular diseases, obesity, etc. Two decades into the twenty-first century, the top worldwide causes of death as reported by the WHO Global Health Observatory are coronary heart disease (also known as ischemic heart disease) and strokes (World Health Organisation (WHO) Global Health Observatory (GHO) Data 2018). In 2012, 2.2 million deaths were attributed to high levels of glucose in the blood, with diabetes another major cause of deaths and disease-affected life years. Since these lifestyle-related health risks that have begun to affect an increasing number of children, it is prudent to consider the dietary components of individuals as a major contributor to the development of these diseases. Estimates made by the Food and Agriculture Organization of the United Nations (FAO) in the year 2015, show an increase in consumption of starch-containing foods around the world due to several factors such as urbanization and rising incomes. The global top five food crops, namely sugarcane, maize, wheat rice, and potatoes, are the most popular choices in diets.
To evaluate the quality of carbohydrate foods to its health impact, there is a scale called the glycemic index (GI). GI is an in vivo physiological measure of the potential of carbohydrate-containing foods to raise blood glucose levels (Jenkins and Wolever 1981). Foods that are classified as having a high GI have a GI value of above 70 e.g. boiled potatoes, cooked rice; those with a low GI have a GI value less than 55 e.g. barley, boiled carrots, and the range between these values belong to medium GI foods e.g. rolled oats, plantains (Ek et al. 2012; Aston et al. 2008). The glycemic index of foods plays a big role in regulating post-prandial blood glucose levels; long-term consumption of high GI foods which are digested and absorbed into the blood rapidly have thus been correlated with various health risks, including obesity, type-2 diabetes mellitus, and cardiovascular diseases. Achieving stable levels of postprandial blood glucose and consequently glycemic control through consumption of resistant starches has been proposed as a mode of treatment for diabetes and pre-diabetes (Wong and Louie 2017). Despite the lack of understanding of the underlying mechanisms, several animal and human studies have shown a link between the consumption of resistant starch and improved glycemic control. Some of the suggested mechanisms are centered around the effects brought about by RS consumption on fat metabolism, diminishing glycemic load, and increasing insulin tolerance (Wong and Louie 2017). Foods can also be measured in terms of satiety, the feeling of content after consuming a meal, or the state of satisfying one’s appetite to the full. The satiety index associated with foods is the ability of the food to satisfy the hunger within a period. High satiety foods are those that enable a person to be sated or ‘filled’ after eating a fixed amount of the food, which prevents the consumption of more calories than intended, thus making such foods healthier alternatives. The relation between glycemic index, satiety, and sugar metabolism can be investigated for healthier alternatives to high-sugar diets. This study involved in vitro digestion of starch samples with enzymes, followed by the analysis of the rate of glucose released from them.
Potato Starch
Potatoes (Solanumtuberosum L.) belong to the tuber family and are a versatile food crop that has been consumed worldwide for centuries since its mass cultivation began nearly six thousand years ago in the South American Andes Mountains (Food and Agriculture Organisation 2008). Since then, several new varieties have been introduced from wild species and spread around the world through various conquests and mass migrations. Currently, they are grown in over 100 countries worldwide and there are thousands of varieties of potatoes cultivated in temperate, tropical and sub-tropical regions of the world (Food and Agriculture Organisation 2008). Over the years, potatoes have been incorporated into diets in various forms, usually after processing with some form of heat during cooking. Today, potatoes have become an indispensable part of diets and economies, especially in developing countries that collectively produce more potatoes than developed ones (Food and Agriculture Organisation 2008). The development of micropropagation techniques has enabled them to be grown devoid of diseases and improved yield. Further advancements in genetic technology have also enabled the production of cultivars that could produce more stable yields with improved nutritional quality, though they have not yet been released for cultivation (Pribylova et al. 2006; Newell-McGloughlin 2008).
Apart from its importance as food, potatoes have extensive uses in industries, mainly due to their starchy nature (Dupuis and Liu 2019). An interesting study conducted by Li et al. (2012) used potato starch for in situ fabrication of hybrid carbon nano-fibers and activated carbon used as the substrate to produce eco-friendly and cost-effective carbon nanotubes, which are desirable features for nanotechnology applications in coming times. Potato starch, like its counterparts from other plant sources, consists of amylose and amylopectin. The composition of amylose varies with different strains of potatoes and has been approximated at 20–33% of the total starch present, while the remainder consists of amylopectin (Liu and Xu 2008). Potato starch in its native form is known to consist of a major percentage of resistant starch (RS), which means that there is always some kind of treatment that potatoes are usually subjected to for ease of digestion, the most common being cooking. The effect of varying pH during hydrolysis of potato starch has been linked to changes in various structural aspects of the starch granules. For example, a study carried out by Kim and Huber (2013) showed that mildly acidic conditions of pH 6 limited the hydrolysis of heat moisture treated potato starch, while a pH of 5 led to lower levels of resistant starch due to a higher degree of granular hydrolysis. This study showed that these mildly acidic conditions facilitated the potato starch to be hydrolyzed within a shorter timeframe. On the other hand, the treatment of potato starch under alkaline conditions followed by structural characterization has shown that its structural and physicochemical properties were modified as a result, where gelatinization temperature increased significantly, and amylose content decreased (Nor Nadiha et al. 2010).
Amylases
Amylases (EC 3.2.1.1,1,4-D-glucanohydrolyase) are a group of enzymes that catalyze the degradation of starch into maltose, maltotriose and other low molecular weight dextrins. Apart from their digestive action in the gut, they are also of great commercial importance in industries ranging from textile, sugar and paper industries to brewing, baking and starch liquefaction to produce glucose and maltose (Lévêque et al. 2000). This makes amylase an enzyme of particular scientific interest. They are metalloenzymes, which means that they contain at least one Ca2+ ion per enzyme molecule (essential for their activity and maintaining structural integrity). Removal of Ca2+ leads to a decline in thermo-stability, enzymatic activity, or both (Buckow et al. 2007). Amylases have been observed to be produced naturally in a variety of sources, from microorganisms such as thermophilic bacteria and the gut of several animals. In humans, two types of amylases are involved in the digestion of carbohydrates; salivary amylase (also known as ptyalin) and pancreatic amylase. The amylase produced by the human pancreas is responsible for the degradation of amylose in consumed carbohydrates by random cleavage of the α-1, 4 glycosidic bonds at the non-terminal ends of the glucose chain (Butterworth et al. 2011). There have been extensive studies carried out to investigate the rate and degree of hydrolysis of potato starch by amylases from different origins. From these studies, it is clear that potato starch exhibits the highest level of resistance to digestion by amylases (Hoover 2001). The surface area to volume ratio is a factor that determines the rate at which amylase digests the starch granules, which is why smaller granules are broken down faster than larger ones, given that the size of potato starch granules varies greatly (diameter between 5 and 150 μm). Another factor that affects the digestibility of starch is the percentage of amylose contained within the starch granule. Higher amylose compositions in starch have been linked to lower digestibility due to resistance, though the exact mechanism is unknown (Ek et al. 2012). Moreover, the length of the amylose chains in potato starch is longer and could contribute to the stability of the overall structure, thereby playing a role in the resistance exhibited by starch molecules.
α-amylases (also known as alpha-1, 4 glucan-4-glucanohydrolase) are a class of hydrolase enzymes that are mainly responsible for in vivo degradation of starch. In humans, starch digestion is initiated by salivary α-amylase in the mouth; the starch is degraded into oligomers by chewing and incorporation of α-amylase with the food, which is further degraded by pancreatic αamylase in the duodenum of the small intestine into maltose, maltotriose and malto-oligosaccharides in the small intestine (Bukhari and Rehman 2015). It has been observed that the activity of pancreatic α-amylase activity is low in infants; for this reason, salivary α-amylase is responsible for the digestion of a large proportion of starch once weaning occurs and the infant begins to consume non-dairy foods. In the case of adults, however, the majority of starch digestion is carried out by pancreatic α-amylase (Butterworth et al. 2011). In humans, there are two main gene groups that code for α-amylases; salivary α-amylase is coded for by the AMY1 genes, which consist of AMY1A, AMY1B and AMY1C genes. Pancreatic α-amylase is coded for by the AMY2 genes, which include the AMY2A and AMY2B genes (Hardy et al. 2015). These are two highly similar gene groups that have approximately 97% sequence similarity (Butterworth et al. 2011). The α-amylases from microorganisms such as bacteria and fungi have been studied intensely due to their ability to resist denaturation at high temperatures, therefore proving to be useful in reactions that require higher temperature optimums and are consequently used for various industrial purposes such as alcohol production, production of sugar, textile and detergent industries (Buckow et al. 2007; Bukhari and Rehman 2015; Shafaat et al. 2011). Examples of micro-organisms include Bacillus subtilis, Bacillus licheniformisandBacillus amyloliquefaciens, among others. Additionally, the optimum pH is acidic, at a value of about 5.5, which is unlike human pancreatic α-amylase (Greenwood and Macgregor 1965). The α-amylase isolated from porcine pancreas has been observed to have the highest homology to the human pancreatic a-amylase among all known amylases studied using molecular cloning and analysis of primary structure (Anitha Gopal and Muralikrishna 2009). Its three-dimensional structure is very similar to that of human pancreatic α-amylase, making it a suitable alternative to using human pancreatic α-amylase for experimental purposes. Amylase (α-amylases and ß-amylase) is present in low amounts in malted grains such as barley and has been fundamental in the process of brewing and preparation of alcoholic beverages, such as beer and whiskey (Buckow et al. 2007). The α-amylase isolated from barley malt has been observed to be sensitive to high temperatures, pressure, salinity and the composition of the medium used during the reaction, therefore requiring the strict maintenance of optimum conditions when carrying out reactions involving this enzyme (Bertoft et al. 1984). ß-amylase (α-1, 4 glucanmaltohydrolase; E.C:3.2.1.2) is a type of exo-amylase that is predominantly found in various plant sources and can also be produced by microbes such as fungi and bacteria but are not found in any animals. Their main function in the digestion of starch is by hydrolysis of α-1, 4 glycosidic bonds (they cannot hydrolyze α-1, 6 glycosidic bonds) to remove maltose units from the non-reducing ends of the polysaccharide chains (Adeyanju et al. 2012). ß-amylase is widely used industrially for the production of maltose syrup from starch, which is a component of various other commercial products.
Characterization of Potato Starch
The characterization is vital in improving understanding of the structural and physicochemical features of the starch under different conditions and to observe how the starch reacts when certain parameters are varied. There have been several studies to characterize the effect that changing temperature and enzyme concentration may have on the structure of starch molecules. This is especially important in industrial applications of starch, where extensive knowledge allows for feasibility in function as well as economically advantageous. It is also helpful from a clinical perspective for patients suffering from diabetes and cardiovascular diseases, as this knowledge will enable them to maintain a diet that is most suitable for their respective conditions. The characterization can be performed in terms of microscopic methods for visualization of the starch granules, while spectroscopic methods aid in understand the broader physicochemical properties of starch.
Optical Microscopy
Optical microscopy is an imaging technique that employs visible light to view a specimen placed under magnifying lenses. It is a simple method of observing and distinguishing potato starch granules clearly, as they are easily visible from a magnification of 40X and undergo minimal photodamage. It can be used to view basic morphological features of the starch granules such as size and shape. Potato starch granules have a distinct spherical or oval shape, consisting of concentric rings of amorphous and crystalline layers of amylose and amylopectin helices originating from a hilum (Kowsik and Mazumder 2018). Clear distinctions can also be made between native and hydrolyzed potato starch granules using higher magnifications on an optical microscope. A study by De Oliveira et al. (2015) demonstrated modifications in structure as a result of high-pressure treatment of tuber starches visible with light microscopies, such as distortion, disruption, swelling and agglomeration of starch granules. The samples also require relatively less preparation, as only a solution is required additionally. However, limitations of this technique arise in higher magnifications, where finer structures are difficult to visualize due to constraints in resolution and contrast of the images; for instance, SEM studies have revealed that potato starch granules are composed of an outer shell structure, which does not resolve clearly with optical microscopy (Chakraborty et al. 2020). To improve contrast, samples can be stained, or polarized light can be employed (polarization microscopy). Figure 1 shows the microscopic images of potato starch granules under bright field, polarized light and confocal laser scanning microscope.
Microscopic images of potato starch granules; (a) Bright field and (b) Polarised light mode. Figure reproduced from Ek et al. 2012 with kind permission from Elsevier. (c) Potato starch images under (c) optical microscopy (d) CLSM images enhanced with a fluorescent dye. Figure reproduced from Han and Hamaker 2002 with kind permission from Elsevier. Optical microscopy images of (e) Native potato starch granules and (f) hydrolyzed potato granules. Figure reproduced from Kowsik and Mazumder 2018 with permission from John Wiley and Sons
Confocal Laser Scanning Microscopy (CLSM)
CLSM is a type of optical scanning technique that involves the use of laser beams to increase contrast and resolution of the image by blocking out unwanted planes of light. Different focal planes can be combined to produce the image, thus enabling viewing of various cross-sections of the sample. It allows optical sectioning of starch granules and eliminates the need for lengthy processing of samples such as drying, embedding and sectioning, which are usually required for scanning electron microscopy (Gray and BeMiller 2004). Additionally, CLSM is useful in understanding the molecular packaging of starch constituents as it enables visualization of starch granule-associated proteins, which are indicators of sites where biosynthesis of amylose occurs. Modified CLSM approaches, such as reflectance confocal laser scanning microscopy (R-CLSM), provide an easier and faster way of studying starch microstructures. Previous studies using R-CLSM have been used to reveal reaction sites and anionic sites in starch granules, including in chemically modified starches (Gray and BeMiller 2004). It has also been employed in previous studies to observe the structural changes to potato starch as it undergoes enzymatic hydrolysis at different temperatures. This has revealed the porous nature of enzyme-hydrolyzed potato starch, which has been suggested to be used as an encapsulant (Apinan et al. 2007). Figure 2 depicts the microscopic images enzyme hydrolyzed potato starch granules under a confocal laser scanning microscope.
CLSM images of potato starch hydrolyzed with α-amylase at varying temperatures and two different units of the enzyme (A1-E1, 165 U/mL, A2-E2, 1522 U/mL) to observe structural changes. The images are reproduced from Apinan et al. 2007 with permission from John Wiley and Sons
Scanning Electron Microscopy (SEM)
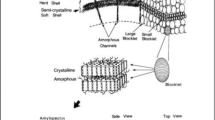
SEM is an effective technique that is used to study the structural features of starch molecules. It is an important microscopy technique that is often used along with optical microscopy for structural characterization of starch; SEM offers higher magnification and resolution as compared to optical microscopy and can provide clearer images of finer morphological details such as grooves and notches on the starch surface (Vafina et al. 2018). It is especially useful to study hydrolyzed starch to view how certain pre-treatments of the starch and enzymatic actions have affected the structure of the granules. Starch processed through other methods such as cross-linking can also be viewed clearly under the SEM field (Martínez et al. 2019). It is important to note that the sizes of individual granules of potato starch also vary minutely with different strains of potatoes, and therefore SEM provides a more accurate method of distinguishing between starches from different potato strains (Martínez et al. 2019). SEM observations of heat-treated and chemically treated starch have been pivotal in the understanding of the three-dimensional structure of individual granules of starch. The blocklet model of the structure of starch was first proposed by Gallant et al. (1997), where a new concept of arrangement of the crystalline molecules amongst the amorphous material in a granule was proposed. This involved the study of starch degraded by pancreatic α-amylase using high magnification under the SEM field, which was subsequently confirmed using atomic force microscopy (AFM). In this model, starch treated with α-amylase was observed to consist of smaller stacked units called ‘blocklets’ within larger, shell-like structures. Fig. 3 depicts the schematic representation of the internal architecture of the starch granule in general. In the case of potato starch which exhibits B-type crystallinity, SEM studies have shown that these blocklets are relatively larger (200–500 nm) and are stacked on top of each other within the granule (Gallant et al. 1997). A detailed characterization study of the outer shell and inner blocklets of native and hydrolyzed potato starch using SEM indicated that the outer shell is flexible and more resistant to shear; the individual blocklets swell during heat-moisture treatment and break out of the outer shell (Huang et al. 2014). Figure 4 depicts the high-resolution images of potato native and hydrolyzed potato starch which are viewed under the scanning electron microscope.
Schematic diagram of potato starch granule showing the internal structural organization of blocklets. The image is reproduced from Huang et al. 2014 with permission from Elsevier
Potato starch granules are observed under high-resolution SEM. (a) Native, (b) Heat-treated shows swollen and distorted granule, (c) The outer shell of broken granule, (d) The outer shell and inner blocklets. The images are reproduced from Huang et al. 2014 with permission from Elsevier
X-Ray Diffraction (XRD)
XRD is a technique that measures the different phases of a crystalline material using X-Rays generated by a cathode ray tube. It is the only technique available to measure the long-range crystalline order of starch (Warren et al. 2016). XRD has been used to study the effects of pre-treatment and enzymatic treatment on potato starch, where changes in crystalline content occur during heating, leading to a change in the maxima obtained after XRD is performed. XRD spectra can be collected over a range of temperatures if the effects of gelatinization are desired; the diffraction pattern can help deduce the crystalline and amorphous areas in starch and therefore calculate its crystallinity (Liu et al. 2002; Bidzińska et al. 2015). Figure 5 shows the XRD spectra of the native and thermally treated potato starch. The X-axis represents the angle of diffraction and the Y-axis is the intensity. XRD patterns significantly reduced as the thermal treatment proceeds. A sharp XRD peak is diminished in the case of curve Fig. 5 (d) which suggests the complete loss of crystallinity at the higher temperature. The orientation of amylose and amylopectin molecules contributes to its characteristic birefringence pattern (also known as the Maltese cross arrangement) and crystallinity (Pielichowski et al. 1998). During the process of gelatinization, starch-water interactions lead to a loss in crystallinity and birefringence of the starch granules, which can be viewed by changes in its XRD patterns (Donovan 1979). Due to the limitations of optical microscopy and FTIR to parameters such as the temperature range and starch-water ratio, X-ray diffraction has been employed to study the transition of starch in a variety of moisture contents and temperature changes (Liu et al. 2002). Comparison of XRD spectra of native potato starch with those of processed ones, such as heat-treated potato starch shows that crystallinity of the starch reduces after microwave and steam treatment (Zhang et al. 2020). The extent of gelatinization also affects the physical properties of the starch; a lower degree of gelatinization of potato starch was associated with lower enthalpy, a faster rate of melting of the starch and formed a more stable gel.
X-ray diffraction patterns of the native and thermally treated potato starch (a) at 210 °C for 30 min, (b) at 210 °C for 2 h, (c) at 230 °C for 30 min, (d) at 230 °C for 2 h. The X-axis represents the angle of diffraction and Y-axis is the intensity. The images are reproduced from Bidzińska et al. 2015 with permission from John Wiley and Sons
Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectroscopy analyses the representative chemical bonds as infrared radiation is passed through a sample. It allows assignment of vibrational bands belonging to particular starch samples to characterize them, such as amylose and amylopectin chain folding and ratios of a crystalline and amorphous material present (Dankar et al. 2018). This way, characteristic peaks in the FTIR spectra can be viewed for each type of potato starch. It is especially useful to study processed starches that are modified industrially, as all possible molecular interactions can be evaluated beforehand. Irradiation, a technique used for preservation of food, alters the physical and nutritional properties of starch in foods, which can also be elucidated by FTIR analysis (Kizil et al. 2002). Comparing the vibrational spectra of native potato starch with that of hydrolyzed potato starch will highlight the changes in chemical bonding that occurs during hydrolysis and other treatments. Moreover, spectral comparisons can be made between native and retrograded starches, and further, analyze the process of retrogradation as it occurs (Dankar et al. 2018). For instance, peaks occurring in the wavenumbers ranging between 3100 and 3700 cm−1 represents the O-H bonds (the alcohol bonds), which are usually present in both native and hydrolyzed potato starch. Similarly, the wavenumber range between 2920 and 2928 cm−1 is representative of CH2 bonds (methylene linkages) in the starch molecules, which are absent in native potato starch but present in hydrolyzed potato starch (Kowsik and Mazumder 2018). Figure 6 represents the FTIR spectra of freeze-dried potato flour. The role of water in the maintenance of structure and recovery of molecular conformation of the starch molecules is a phenomenon that can be studied through FTIR. In an investigation conducted by Dankar et al., it was reported that both dehydrated and hydrated potato starch samples both presented the peak at 1650 cm−1, which corresponds to water absorbed in the amorphous regions of starch. Both vibrational spectra were similar, except for reduced intensity in the hydrated samples and an additional peak at 2100 cm−1, which was assigned to the free water content present in the hydrated samples. This occurrence has been hypothesized to be a result of water molecules having a structural role in the starch molecules (Dankar et al. 2018). Processing of potato starch utilizing high temperatures and shear has also been linked to the deformation of O-H bonds, which consequently affect its structural properties (Zhang et al. 2020).
FTIR spectrum of potato starch before and after pasting showing characteristic peaks. The peak between 3800 and 3000 cm−1 represents O–H bond stretching, while the peaks at ~1082 cm−1 represent C–H bond bending. The image is reproduced from Zhang et al. 2020
Differential Scanning Calorimetry (DSC)
DSC is a thermo-analytical method of measuring the heat energy change that occurs in a sample of starch when it undergoes gelatinization, a process where starch is heated in the presence of excess water. It measures the change in enthalpy that occurs when the hydrogen bonds in the starch molecules are broken, leading to a change in molecular order (Ek et al. 2012). This value depends on the crystallinity of the starch molecules (Tester et al. 1993). The structure of native starch consists of large, semi-crystalline granules that have several amorphous regions. The main enzyme involved in starch digestion in humans is α-amylase, which is affected by the presence of other artefacts such as lipids and proteins on the starch molecules. The crystalline regions of starch have also been shown to be unfavorable for attack by the much smaller enzyme molecules. The length and branching points of the amylose and amylopectin molecules packed into double helices bonded by hydrogen determine the crystallinity of the native starch molecules from each plant source. Crystallinity of starch is generally categorized into two types; A-type consists of orthogonally arranged helices while B-type consists of hexagonally arranged helices. Generally, starch from cereals such as rice and wheat tend to have A-type crystallinity while the starch from tubers such as potatoes tends to have B-type crystallinity. These factors affect how amylases digest the starch; native potato starch, which has a higher B-type crystallinity is less susceptible to digestion by amylases. Subjecting native starch to hydrothermal treatments of varying degrees affects its crystalline structure to be disrupted in a process known as solubilization, therefore increasing its digestibility by amylases (Butterworth et al. 2011). This kind of treatment is analogous to the cooking of the potatoes before consumption, as uncooked potatoes contain a large percentage of indigestible native resistant starch.
When starch is further subjected to hydrothermal treatment beyond a particular temperature, the structural changes associated with the starch molecules are different due to the formation of amorphous structures with the starch molecules. This is due to the phenomenon that starch subjected to hydrothermal treatments (also known as heat moisture treatment or HMT) can be done in a manner such that the structure of the starch is preserved while modifying its physiochemical properties (De Oliveira et al. 2018). Above a particular temperature, potato starch has been observed to lose its crystallinity and forms an insoluble, gel-like substance in a process known as gelatinization. This temperature, also known as the gelatinization temperature, is determined by DSC curves (Lee et al. 2011) and has been observed for potato starch to be at a range between 58.8 °C (onset temperature) and 72.0 °C (end temperature) with a peak temperature of 64.4 °C (Liu et al. 2002). Gelatinization enthalpies for potato starch are generally between the ranges from 18.5 to 19.5 Jg−1 (Ek et al. 2014). If this starch is cooled, these disrupted structures can reform themselves back into crystalline structures, forming an insoluble material that resists digestion by amylases. This process is known as retrogradation of starch (Butterworth et al. 2011). Gelatinization and retrogradation of starch are processes that are of importance during the processing of starch products, thus establishing the significance of conducting extensive studies in this regard.
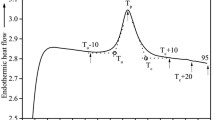
Studies have been carried out to investigate the effect of various forms of commonly used heat treatments on resistant starch-containing foods. It has been shown that some cooking methods, such as steaming, baking and autoclave cooking led to an increase in the amount of resistant starch in the food. On the other hand, other forms of cooking such as pressure cooking led to a decrease in the resistant starch content in the food, whereas methods such as frying, microwaving, boiling and extrusion cooking affect the starch in different ways depending on the starch source and the conditions present during processing (Elif and Zehra 2017). The techniques employed to process starch, such as temperature, extrusion and tempering have varying effects on the resistant starch present in a food, consequently having varied physiological effects in the body. For foods such as cereals and grains which contain RS1, the extent of milling affects the starch digestion as the high shear process fragments the non-digestible outer matrix in which the starch granules are located. Heat-moisture treatments that result in gelatinization followed by retrogradation lead to an increase in the RS3 content of foods as a result of changes in moisture, heating and cooling (Alsaffar 2011). Additionally, storage conditions and time taken for heating and cooling also influence the resistant starch ratios in processed foods; in boiled potatoes, it was observed that cooling for 24 h can further affect the RS content and subsequently its glycemic response when digested in vitro (Garcia-Alonso and Goni 2000). Studies performed on the kinetics of the gelatinization of potato starch enables a better understanding of the structure of starch and therefore improving its range of applications. Starch is known to consist of linear amylose and branched amylopectin arranged within its amorphous and crystalline regions (Pielichowski et al. 1998). This arrangement influences the endothermic reactions that accompany the gelatinization of starch in the presence of sufficient water, which can be observed using DSC (as shown in Fig. 7).
DSC thermograms of potato starch heated at a rate of 10 °C/min labeled with a volume fraction of water present. The image is reproduced from Donovan 1979 with permission from John Wiley and Sons
To discuss the process of gelatinization, it may be classified into three nearly indistinguishable phases: (a) the starch granules lose its crystallinity; (b) conformational changes occur in the starch structure through the uptake of heat energy and (c) the starch granule swells as it absorbs water (Donovan 1979) (as shown in Fig. 7). The gelatinization temperature is represented by the maximum peak position attained in the DSC curve. The amount of water present while the starch sample is heated will affect the peak gelatinization temperature, which can be viewed as different endotherms in the resulting DSC curve. When heated with sufficient water, Hollinger et al. reported a single endotherm value to occur at 66 °C, referred to as the gelatinization phase change. The study of gelatinization parameters using DSC also enables inference of the amylose content in the starch samples; in essence, the enthalpy change of gelatinization is higher in starches with higher amylose content as compared to those with lower amylose content, given that both starches have equal crystallinity patterns. The reason for this thermal behavior is due to amylose being more associated in solution than amylopectin, therefore enabling higher levels of interaction with the solution (Hollinger et al. 1974).
Conclusion and Prospects
Potatoes are a multi-faceted food which invariably accounts for a significant portion of daily diets, while potato starch remains a versatile industrial component as the most commonly used food additive. While its applications move beyond the food industry to other novel prospects such as nanotechnology and tissue engineering, its nutritional importance makes it a highly researched biopolymer. This is especially important in diet planning for individuals with diseases such as diabetes, chronic heart disease and colon cancer, as the glycemic index of potato starch varies when subjected to different conditions. Characterization using various microscopic and spectroscopic methods broadens knowledge on the behavior of potato starch under different conditions, such as heat-moisture treatment, cold storage and crosslinking. Microscopic methods to study structural properties include but are not limited to optical microscopy, CLSM and SEM. Spectroscopic techniques provide insight into the physical features and properties of potato starch, with X-ray diffraction used to understand crystallinity, FTIR evaluating the chemical bonds and DSC to study how the properties of starch vary when an enthalpy change occurs. Most studies that were conducted to date involve in vitro assessment of digestibility and assessment by structural characterization. This can be used as the basis for future projects to conduct in vivo studies to determine the effect of digestive enzymes on treated potato starch to assess how glycemic index varies accordingly. Additionally, potato starch can be characterized in terms of its amylose and amylopectin content using chromatography. This enables revealing of new applications of potato starch and further promotes research into its properties.
References
Acquarone, V.M., and M.A. Rao. 2003. Influence of sucrose on the rheology and granule size of cross-linked waxy maize starch dispersions heated at two temperatures. Carbohydrate Polymers 51: 451–458. https://doi.org/10.1016/S0144-8617(02)00217-5.
Adeyanju, M.M., Adetoro, A.O., Adeshakin, A.O., Kasumu, T., Mowoe, O., Famakinwa, O.A., and Lawal, O. 2012. Characterization of a thermostable Bacillus subtilis ßamylase isolated from decomposing peels of cassava (Manihotesculenta). Biokemistri24.
Alsaffar, A.A. 2011. Effect of food processing on the resistant starch content of cereals and cereal products – A review. International Journal of Food Science & Technology 46: 455–462. https://doi.org/10.1111/j.1365-2621.2010.02529.x.
Anitha Gopal, B., and G. Muralikrishna. 2009. Porcine pancreatic α-amylase and its isoforms: Purification and kinetic studies. International Journal of Food Properties 12: 571–586. https://doi.org/10.1080/10942910801947755.
Apinan, S., I. Yujiro, Y. Hidefumi, F. Takeshi, P. Myllärinen, P. Forssell, and K. Poutanen. 2007. Visual observation of hydrolyzed potato starch granules by α-amylase with confocal laser scanning microscopy. Starch -Stärke 59: 543–548. https://doi.org/10.1002/star.200700630.
Aston, L.M., J.M. Gambell, D.M. Lee, S.P. Bryant, and S.A. Jebb. 2008. Determination of the glycemic index of various staple carbohydrate-rich foods in the UK diet. European Journal of Clinical Nutrition 62: 279–285. https://doi.org/10.1038/sj.ejcn.1602723.
Bao, J. 2017. Starch in health and disease. Starch-Stärke, 69:1770076. https://doi.org/10.1002/star.201770076.
Bertoft, E., C. Andtfolk, and S. Kulp. 1984. Effect of pH, temperature, and calcium ions on barley malt α-amylase isoenzymes. Journal of the Institute of Brewing 90: 298–302. https://doi.org/10.1002/j.2050-0416.1984.tb04278.x.
Bidzińska, E., M. Michalec, and D. Pawcenis. 2015. Effect of thermal treatment on potato starch evidenced by EPR, XRD and molecular weight distribution. Magnetic Resonance in Chemistry 53: 1051–1056. https://doi.org/10.1002/mrc.4310.
Brownlee, I.A., S. Gill, M.D. Wilcox, J.P. Pearson, and P.I. Chater. 2018. Starch digestion in the upper gastrointestinal tract of humans. Starch-Stärke 70: 9–10. https://doi.org/10.1002/star.20170011.
Buckow, R., U. Weiss, V. Heinz, and D. Knorr. 2007. Stability and catalytic activity of alpha-amylase from barley malt at different pressure-temperature conditions. Biotechnology and Bioengineering 97: 1–11. https://doi.org/10.1002/bit.21209.
Bukhari, D., and A. Rehman. 2015. Purification and characterization of a-amylase from Bacillus subtilis isolated from local environment. Pakistan Journal of Zoology 47: 905–911.
Butterworth, P., F. Warren, and P. Ellis. 2011. Human alpha-amylase and starch digestion: An interesting marriage. Starch-Starke 63: 395–405. https://doi.org/10.1002/star.201000150.
Chakraborty, I., S. Pallen, Y. Shetty, N. Roy, and N. Mazumder. 2020. Advanced microscopy techniques for revealing molecular structure of starch granules. Biophysical Reviews 12: 105–122. https://doi.org/10.1007/s12551-020-00614-7.
Dankar, I., Haddarah, A., Omar, F.E.L., Pujolà, M., and Sepulcre, F. 2018. Characterization of food additive-potato starch complexes by FTIR and X-ray diffraction. Food Chemistry 260. Elsevier: 7–12. https://doi.org/10.1016/J.FOODCHEM.2018.03.138.
De Oliveira, M.M., A.A.L. Tribst, B.R. de LeiteJúnior, C. Oliveira, R.A. de, and M. Cristianini. 2015. Effects of high pressure processing on cocoyam, Peruvian carrot, and sweet potato: Changes in microstructure, physical characteristics, starch, and drying rate. Innovative Food Science and Emerging Technologies 31: 45–53. https://doi.org/10.1016/j.ifset.2015.07.004.
De Oliveira, C.S., C.D. Bet, R.Z.B. Bisinella, L.H. Waiga, T.A.D. Colman, and E. Schnitzler. 2018. Heat-moisture treatment (HMT) on blends from potato starch (PS) and sweet potato starch (SPS). Journal of Thermal Analysis and Calorimetry, 133:1491–1498. https://doi.org/10.1007/s10973-018-7196-9.
Donovan, J.W. 1979. Phase transitions of the starch–water system. Biopolymers 18: 263–275. https://doi.org/10.1002/bip.1979.360180204.
Drummond, G.S., E.E. Smith, and W.J. Whelan. 1971. A general method for distinguishing between endo and exo actions of carbohydrases. FEBS Letters 15: 302–304. https://doi.org/10.1016/0014-5793(71)80643-9.
Dupuis, J., and Q. Liu. 2019. Potato starch: A review of physicochemical, functional and nutritional properties. American Journal of Potato Association 96: 127–138. https://doi.org/10.1007/s12230-018-09696-2.
Ek, K.L., J. Brand-Miller, and L. Copeland. 2012. Glycemic effect of potatoes. Food Chemistry 133: 1230–1240. https://doi.org/10.1016/j.foodchem.2011.09.004.
Ek, K., S. Wang, J. Brand-Miller, and L. Copeland. 2014. Properties of starch from potatoes differing in glycemic index. Food & Function 5: 2509–2515. https://doi.org/10.1039/c4fo00354c.
Elif, I.E., and B. Zehra. 2017. The effect of various cooking methods on resistant starch content of foods. Nutrition andFood Science 47: 522–533. https://doi.org/10.1108/NFS-10-2016-0154.
Englyst, H.N., S.M. Kingman, G.J. Hudson, and J.H. Cummings. 1996. Measurement of resistant starch in vitro and in vivo. British Journal of Nutrition 75: 749–755. https://doi.org/10.1079/BJN19960178.
Escarpa, A., M.C. González, M.D. Morales, and F. Saura-Calixto. 1997. An approach to the influence of nutrients and other food constituents on resistant starch formation. Food Chemistry 60: 527–532. https://doi.org/10.1016/S0308-8146(97)00025-3.
Food and Agriculture Organisation 2008. International year of the Potato (Potato World) http://www.fao.org/potato-2008/en/world/. Accessed 15 February 2020.
Gallant, D.J., B. Bouchet, and P.M. Baldwin. 1997. Microscopy of starch: Evidence of a new level of granule organization. Carbohydrate Polymers 32: 177–191. https://doi.org/10.1016/S0144-8617(97)00008-8.
Garcia-Alonso, A., and I. Goni. 2000. Effect of processing on potato starch: In vitro availability and glycemic index. Nahrung 44: 19–22. https://doi.org/10.1002/(SICI)15213803(20000101)44:1<19::AIDFOOD19>3.0.CO;2-E.
Gray, J.A., and J.N. BeMiller. 2004. Development and utilization of reflectance confocal laser scanning microscopy to locate reaction sites in modified starch granules. Cereal Chemistry 81: 278–286. https://doi.org/10.1094/CCHEM.2004.81.2.278.
Greenwood, C.T., and A.W. Macgregor. 1965. The isolation of α-amylase from barley and malted barley, and a study of the properties and action-patterns of the enzymes. Journal of the Institute of Brewing 71: 405–417. https://doi.org/10.1002/j.2050-0416.1965.tb06366.x.
Han, X.-Z., and B.R. Hamaker. 2002. Location of starch granule-associated proteins revealed by confocal laser scanning microscopy. Journal of Cereal Science 35: 109–116. https://doi.org/10.1006/jcrs.2001.0420.
Hardy, K., J. Brand-Miller, K.D. Brown, M.G. Thomas, and L. Copeland. 2015. The importance of dietary carbohydrate in human evolution. The Quarterly Review of Biology 90: 251–268. https://doi.org/10.1086/682587.
Hollinger, G., L. Kuniak, R.H. Marchessault, and R.H. Marchessault. 1974. Thermodynamic aspects of the gelatinization and swelling of crosslinked starch. Biopolymers 13: 879–890. https://doi.org/10.1002/bip.1974.360130504.
Hoover, R. 2001. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydrate Polymers 45: 253–267. https://doi.org/10.1016/S0144-8617(00)00260-5.
Huang, J., N. Wei, H. Li, S. Liu, and D. Yang. 2014. Outer shell, inner blocklets, and granule architecture of potato starch. Carbohydrate Polymers 103: 355–358. https://doi.org/10.1016/j.carbpol.2013.12.064.
Jenkins, D.J.A., and T.M.S. Wolever. 1981. Slow release carbohydrate and the treatment of diabetes. Proceedings of the Nutrition Society 40: 227–235. https://doi.org/10.1079/PNS19810033.
Kim, J.-Y., and K.C. Huber. 2013. Heat–moisture treatment under mildly acidic conditions alters potato starch physicochemical properties and digestibility. Carbohydrate Polymers 98: 1245–1255. https://doi.org/10.1016/j.carbpol.2013.07.013.
Kizil, R., J. Irudayaraj, and K. Seetharaman. 2002. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. Journal of Agricultural and Food Chemistry American Chemical Society 50: 3912–3918. https://doi.org/10.1021/jf011652p.
Kowsik, P.V., and N. Mazumder. 2018. Structural and chemical characterization of rice and potato starch granules using microscopy and spectroscopy. Microscopy Research and Technique 81: 1533–1540. https://doi.org/10.1002/jemt.23160.
Lee, C.J., S.I. Shin, Y. Kim, H.J. Choi, and T.W. Moon. 2011. Structural characteristics and glucose response in mice of potato starch modified by hydrothermal treatments. Carbohydrate Polymers 83: 1879–1886. https://doi.org/10.1016/j.carbpol.2010.10.057.
Lévêque, E., Š. Janeček, B. Haye, and A. Belarbi. 2000. Thermophilic archaeal amylolytic enzymes. Enzyme and Microbial Technology 26: 3–14. https://doi.org/10.1016/S0141-0229(99)00142-8.
Li, Q., F. Liu, L. Zhang, B.J. Nelson, S. Zhang, C. Ma, X. Tao, J. Cheng, and X. Zhang. 2012. In situ construction of potato starch based carbon nanofiber/activated carbon hybrid structure for high-performance electrical double layer capacitor. Journal of Power Sources 207: 199–204. https://doi.org/10.1016/j.jpowsour.2012.01.142.
Liu, X.D., and Y. Xu. 2008. A novel raw starch digesting α-amylase from a newly isolated Bacillus sp. YX-1: Purification and characterization. Bioresource Technology 99: 4315–4320. https://doi.org/10.1016/j.biortech.2007.08.040.
Liu, Q., G. Charlet, S. Yelle, and J. Arul. 2002. Phase transition in potato starch–water system I. starch gelatinization at high moisture level. Food Research International 35: 397–407. https://doi.org/10.1016/S0963-9969(01)00134-X.
Marquez, G., and M.C. Anon. 1986. Influence of reducing sugars and amino acids in the color development of fried potatoes. Journal of Food Science 51: 157–160. https://doi.org/10.1111/j.1365-2621.1986.tb10859.x.
Martínez, P., F. Peña, L.A. Bello-Pérez, C. Núñez-Santiago, H. Yee-Madeira, and C. Velezmoro. 2019. Physicochemical, functional and morphological characterization of starches isolated from three native potatoes of the Andean region. Food Chemistry: X 2: 100030. https://doi.org/10.1016/J.FOCHX.2019.100030.
Miranda, M.L., and J.M. Aguilera. 2006. Structure and texture properties of fried potato products. Food Reviews International 22: 173–201. https://doi.org/10.1080/87559120600574584.
Morrell, S., and T. Ap Rees. 1986. Control of the hexose content of potato tubers. Phytochemistry 25: 1073–1076. https://doi.org/10.1016/S0031-9422(00)81556-3.
Newell-McGloughlin, M. 2008. Nutritionally improved agricultural crops. Plant physiology. American Society of Plant Biologists 147: 939–953. https://doi.org/10.1104/pp.108.121947.
Nor Nadiha, M.Z., A. Fazilah, R. Bhat, and A.A. Karim. 2010. Comparative susceptibilities of sago, potato and corn starches to alkali treatment. Food Chemistry 121: 1053–1059. https://doi.org/10.1016/j.foodchem.2010.01.048.
Pielichowski, K., Tomasik, P., and Sikora, M. 1998. Kinetics of gelatinization of potato starch studied by non-isothermal DSC. Carbohydrate Polymers 35. Elsevier: 49–54. https://doi.org/10.1016/S0144-8617(97)00236-1.
Pribylova, R., I. Pavlik, and M. Bartoš. 2006. Genetically modified potato plants in nutrition and prevention of diseases in humans and animals: A review. Veterinarni Medicina 51: 212–223. https://doi.org/10.17221/5540-VETMED.
Rodrigues, A., and M. Emeje. 2012. Recent applications of starch derivatives in nanodrug delivery. Carbohydrate Polymers 87: 987–994. https://doi.org/10.1016/j.carbpol.2011.09.044.
Saliu, O.D., G.A. Olatunji, A.I. Olosho, A.G. Adeniyi, Y. Azeh, F.T. Samo, D.O. Adebayo, and O.O. Ajetomobi. 2019. Barrier property enhancement of starch citrate bioplastic film by an ammonium-thiourea complex modification. Journal of Saudi Chemical Society 23: 141–149. https://doi.org/10.1016/j.jscs.2018.06.004.
Sasaki, T., I. Sotome, and H. Okadome. 2015. In vitro starch digestibility and in vivo glucose response of gelatinized potato starch in the presence of non-starch polysaccharides. Starch-Stärke 67: 415–423. https://doi.org/10.1002/star.201400214.
Shafaat, S., M. Akram, and A. Rehman. 2011. Isolation and characterization of a thermostable alpha-amylase from Bacillus subtilis. African Journal of Microbiology Research 5: 3334–3338. https://doi.org/10.5897/AJMR11.666.
Sowokinos, J.R. 2001. Biochemical and molecular control of cold-induced sweetening in potatoes. American Journal of Potato Research 78: 221–236. https://doi.org/10.1007/BF02883548.
Tester, R.F., W.R. Morrison, and A.H. Schulman. 1993. Swelling and gelatinization of cereal starches. V. Risø Mutants of Bomi and Carlsberg II Barley Cultivars. Journal of Cereal Science 17: 1–9. https://doi.org/10.1006/jcrs.1993.1001.
Vafina, A., Proskurina, V., Vorobiev, V., Evtugin, V.G., Egkova, G., and Nikitina, E. 2018. Physicochemical and morphological characterization of potato starch modified by bacterial amylases for food industry applications. Journal of Chemistry1627540. https://doi.org/10.1155/2018/1627540.
Van der Maarel, M.J.E.C., B. van der Veen, J.C.M. Uitdehaag, H. Leemhuis, and L. Dijkhuizen. 2002. Properties and applications of starch-converting enzymes of the αamylase family. Journal of Biotechnology 94: 137–155. https://doi.org/10.1016/S0168-1656(01)00407-2.
Wang, S., and L. Copeland. 2013. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food and Function. 4: 1564–1580. https://doi.org/10.1039/c3fo60258c.
Warren, F.J., M.J. Gidley, and B.M. Flanagan. 2016. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydrate Polymers 139: 35–42. https://doi.org/10.1016/J.CARBPOL.2015.11.066.
Wong, T.H.T., and J.C.Y. Louie. 2017. The relationship between resistant starch and glycemic control: A review on current evidence and possible mechanisms. Starch -Stärke 69: 7–8. https://doi.org/10.1002/star.201600205.
World Health Organisation (WHO) Global Health Observatory (GHO) Data 2018. https://www.who.int/gho/publications/world_health_statistics/2018/en/. Accessed on 10 March 2020.
Zhang, K., Y. Tian, C. Liu, and W. Xue. 2020. Effects of temperature and shear on the structural, thermal and pasting properties of different potato flour. BMC Chemistry 14: 1–8. https://doi.org/10.1186/s13065-020-00670-w.
Zyzak, D.V., R.A. Sanders, M. Stojanovic, D.H. Tallmadge, B.L. Eberhart, D.K. Ewald, D.C. Gruber, T.R. Morsch, M.A. Strothers, G.P. Rizzi, and M.D. Villagran. 2003. Acrylamide formation mechanism in heated foods. Journal of Agricultural and Food Chemistry 51: 4782–4787. https://doi.org/10.1021/jf034180i.
Acknowledgements
We thank the Department of Science and Technology (DST) and Department of Biotechnology (DBT), Government of India, for the financial support (project number: DST/INT/Thai/P-10/2019 and BT/PR25099/NER/95/1014/2017). The authors thank Dr. K. Satyamoorthy, Director, Manipal School of Life Sciences (MSLS), Manipal Academy of Higher Education (MAHE), for his encouragement and MAHE, Manipal, India for providing the infrastructure and facilities. We thank Dr. K. K. Mahato, HoD, Department of Biophysics, MSLS, MAHE, for the fruitful discussion in preparation for this review article.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jagadeesan, S., Govindaraju, I. & Mazumder, N. An Insight into the Ultrastructural and Physiochemical Characterization of Potato Starch: a Review. Am. J. Potato Res. 97, 464–476 (2020). https://doi.org/10.1007/s12230-020-09798-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-020-09798-w