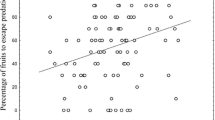
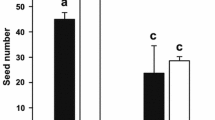
Abstract
Flower size is a key trait in the reproductive ecology of animal-pollinated plants. However, pollinator-mediated selection does not always modulate this trait and environmental conditions and/or antagonist interactions may favor smaller flowers. We evaluate the occurrence of a large-flowered family in a hot and dry Mediterranean environment, mediated by a cost-benefit balance and a male–female conflict. Large flowers have sizeable benefits in terms of pollination and reproductive success and pollinators mediate selection through male function, but female fitness is context-dependent. High floral production and maintenance costs and florivore incidence in large flowers limit female function, which counteracts pollinator-mediated selection. Large flowers are highly costly in the Mediterranean and flower size is mediated by a sexual conflict between the benefits of male function and the costs of the female one. However, a short floral longevity, occasional pollen limitation and selection through maleness keep the existence of large flowers in these environments.







Similar content being viewed by others
Literature Cited
Ågren, J. 1988. Sexual differences in biomass and nutrient allocation in the dioecious Rubus chamaemorus. Ecology 69: 962–973.
Aigner, P. A. 2005. Variation in pollination performance gradients in a Dudleya species complex, can generalization promote floral divergence? Functional Ecology 19: 681–689.
Althoff, D. M., W. Xiao, S. Sumoski & K. A. Segraves. 2013. Florivore impacts on plant reproductive success and pollinator mortality in an obligate pollination mutualism. Oecologia 173: 1345–1354.
Andersson, S. 1988. Size-dependent pollination efficiency in Anchusa officinalis (Boraginaceae): causes and consequences. Oecologia 76: 125–130.
——— 1999. The cost of floral attractants in Achillea ptarmica (Asteraceae), evidence from a ray removal experiment. Plant Biology 1: 569–572.
——— 2000. The costs of flowers of Nigella degenii inferred flower and perianth removal experiments. International Journal of Plant Sciences 16: 903–908.
——— 2001. Fitness consequences of floral variation in Senecio jacobaea (Asteraceae): evidence from a segregating hybrid populations and a resource manipulation experiment. Biological Journal of the Linnean Society 74: 17–24.
——— 2005. Floral costs in Nigella sativa (Ranuncualceae): compensatory responses to perianth removal. American Journal of Botany 92: 279–283.
——— & B. Widén. 1993. Pollinator-mediated selection on floral traits in a synthetic population of Senecio intergrifolius (Asteraceae). Oikos 66: 72–79.
Aragón, C. F. & A. Escudero. 2008. Mating system of Helianthemum squamatum (Cistaceae), a gypsophile specialist of semi-arid Mediterranean environments. Botanica Helvetica 118: 129–137.
———, ——— & F. Valladares. 2008. Stress-induced dynamic adjustments of reproduction differentially affect fitness components of a semi-arid plant. Journal of Ecology 96: 222–229.
———, M. Méndez & A. Escudero. 2009. Survival costs of reproduction in a short-lived perennial plant: live hard, die young. American Journal of Botany 96: 904–911.
Arista, M. & P. L. Ortiz. 2007. Differential gender selection on floral size: an experimental approach using Cistus salviifolius. Journal of Ecology 95: 973–982.
Arrington, J. M. & K. Kubitzki. 2003. Cistaceae. Pp 62–70. In: K. Kubitzki, C. Bayer, & P. F. Stevens (eds). The families and genera of vascular plants vol. V. Springer, Berlin.
Ashman, T.-L. & C. J. Majestic. 2006. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96: 343–352.
——— & M. T. Morgan. 2004. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proceedings of the Royal Society of London B Series 271: 553–559.
——— & D. J. Schoen. 1994. How long should flowers live? Nature 371: 788–791.
——— & ———. 1997. The cost of floral longevity in Clarkia tembloriensis: an experimental investigation. Evolutionary Ecology 11: 289–300.
Atkin, O. K. & M. G. Tjoelker. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8: 343–351.
Barrett, S. C. H., L. D. Harder & W. W. Cole. 2004. Correlated evolution of floral morphology and mating-type frequencies in a sexually polymorphic plant. Evolution 58: 964–975.
Barrio, M. & A. L. Teixido. 2015. Sex-dependent selection on flower size in a large-flowered Mediterranean species: an experimental approach with Cistus ladanifer. Plant Systematics and Evolution 301: 113–124.
Bell, G. 1985. On the function of flowers. Proceedings of the Royal Society of London B Series 224: 223–265.
Blionis, G. J., J. M. Halley & D. Vokou. 2001. Flowering phenology of Campanula on Mt Olympos, Greece. Ecography 24: 696–706.
Blondel, J. & J. Aronson. 1999. Biology and wildlife of the Mediterranean region. Oxford University Press, Oxford.
Bosch, J. 1992. Floral biology and pollinators of three co-occurring Cistus species (Cistaceae). Botanical Journal of the Linnean Society 109: 39–55.
Brandt, U. & G. Gottsberger. 1988. Flower phenology, pollinating insects and breeding systems in Cistus, Halimium and Tuberaria species in Portugal. Lagascalia 15: 625–634.
Brody, A. K. & R. J. Mitchell. 1997. Effects of experimental manipulation of inflorescence size on pollination and pre-dispersal seed production in the hummingbird-pollinated plant Ipomopsis aggregata. Oecologia 110: 86–93.
Brothers, A. N. & J. W. Atwell. 2014. The role of pollinator-mediated selection in the divergence of floral traits between two closely related plant species. International Journal of Plant Sciences 175: 287–295.
Burd, M. & H. S. Callahan. 2000. What does the male function hypothesis claim? Journal of Evolutionary Biology 13: 735–742.
Caballero, I., J. M. Olano, A. L. Luzuriaga & A. Escudero. 2005. Spatial coherence between seasonal seed banks in a semi-arid gypsum community: density changes but structure does not. Seed Science Research 15: 153–160.
Campbell, D. R. & J. M. Powers. 2015. Natural selection on floral morphology can be influenced by climate. Proceedings of the Royal Society of London B Series 282: 20150178.
Cuautle, M. & J. N. Thompson. 2010. Diversity of floral visitors to sympatric Lithophragma species differing in floral morphology. Oecologia 2010: 71–80.
Cardel, Y. J. & S. Koptur. 2010. Effects of florivory on the pollination of flowers: an experimental study with a perennial plant. International Journal of Plant Sciences 171: 283–292.
Carroll, A. B., S. G. Pallardy & C. Galen. 2001. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae). American Journal of Botany 88: 438–446.
Caruso, C. M. 2006. Plasticity of inflorescence traits in Lobellia siphilitica (Lobeliaceae) in response to soil water availability. American Journal of Botany 93: 531–538.
———, D. L. D. Remington & K. E. Ostergren. 2005. Variation in resource limitation of plant reproduction influences natural selection on floral traits of Asclepias syriaca. Oecologia 146: 68–76.
Chapin, F. S., III. 1989. The cost of tundra plant structures: evaluation of concepts and currencies. American Naturalist 133: 1–19.
Chapman, T. 2006. Evolutionary conflicts of interest between males and females. Current Biology 16: 744–754.
Conner, J. K. 2006. Ecological genetics of floral evolution. Pp 260–277. In: L. D. Harder & S. C. H. Barrett (eds). Ecology and evolution of flowers. Oxford University Press, Oxford, UK.
——— & S. Rush. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 105: 509–516.
Cruden, R. W. & D. L. Lyon. 1985. Patterns of biomass allocation to male and female functions in plants with different mating system. Oecologia 66: 299–306.
Darwin, C. 1862. On the various contrivances by which British and foreign orchids are fertilized by insects. John Murray, London, UK.
——— 1877. The different forms of flowers on plants of the same species. John Murray, London, UK.
De la Barrera, E. & P. S. Nobel. 2004a. Nectar: properties, floral aspects, and speculations on origin. Trends in Plant Science 9: 65–69.
——— & ———. 2004b. Carbon and water relations for developing fruits of Opuntia ficus-indica (L.) Miller, including effects of drought and gibberellic acid. Journal of Experimental Botany 55: 719–729.
Duhme, F. & T. M. Hinckley. 1992. Daily and seasonal ariation in water relations of macchia shrubs and trees in France (Montpellier) and Turkey (Antalya). Vegetatio 100: 185–198.
Elle, E. & J. D. Hare. 2002. Environmentally induced variation in floral traits affects the mating system in Datura wrightii. Functional Ecology 16: 79–88.
Erickson, A. N. & A. H. Markhart. 2002. Flower development stage and organ sensivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant Cell and Environment 25: 123–130.
Fang, X., N. C. Turner, G. Yan, F. Li & K. H. M. Siddique. 2010. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. Journal of Experimental Botany 61: 335–345.
Fenster, C. B., W. S. Armbruster, P. Wilson, M. R. Dudash & J. D. Thomson. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics 35: 375–403.
Fleming, T. H., C. T. Sahley, J. Nathaniel-Holland, J. D. Nason & J. L. Hamrick. 2001. Sonoran desert columnar cacti and the evolution of generalized pollination systems. Ecological Monographs 71: 511–530.
Galen, C. 1989. Measuring pollinator-mediated selection on morphometric floral traits, bumblebees and the alpine sky pilot, Polemonium viscosum. Evolution 43: 882–890.
——— 1996. Rates of floral evolution: adaptation to bumblebee pollination in an alpine wildflower, Polemonium viscosum. Evolution 50: 120–125.
——— 1999. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience 49: 631–640.
——— 2000. High and dry, drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). American Naturalist 156: 72–83.
——— 2005. It never rains but then it pours: the diverse effects of water on flower integrity and function. Pp 77–95. In: E. Reekie & F. A. Bazzaz (eds). Reproductive allocation in plants. Elsevier Academic Press, San Diego, USA.
———, T. E. Dawson & M. L. Stanton. 1993. Carpels as leaves: meeting the carbon cost of reproduction in an alpine buttercup. Oecologia 95: 187–193.
———, R. A. Sherry & A. B. Carroll. 1999. Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia 118: 461–470.
Gómez, J. M. 2003. Herbivory reduces the strength of pollinator-mediated selection in the Mediterranean herb Erysinum mediohispanicum: consequences for plant specialization. American Naturalist 162: 242–256.
———, F. Perfectti & J. Lorite. 2015. The role of pollinators in floral diversification in a clade of generalist flowers. Evolution 69: 863–878.
Gulmon, S. L. & H. A. Mooney. 1986. Costs of defence and their effects on plant productivity. Pp 681–698. In: T. J. Givnish (ed). On the Economy of Plant Form and Function. Cambridge University Press, London, UK.
Guzmán, B., E. Narbona & P. Vargas. 2011. Similar reproductive success of the two petal colour polymorphisms of Cistus ladanifer (Cistaceae). Plant Biosystems 145: 931–937.
———, ——— & ———. 2015. Investigating reproductive incompatibility barriers in a Mediterranean rockrose (Cistus ladanifer). Plant Biosystems 149: 1–6.
Halpern, S. L., L. S. Adler & M. Wink. 2010. Leaf herbivory and drought stress affect floral attractive and defensive traits in Nicotiana quadrivalvis. Oecologia 163: 961–971.
Herrera, C. M. 1993. Selection on floral morphology and environmental determinants of fecundity in a hawk moth-pollinated violet. Ecological Monographs 63: 251–275.
——— 2000. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology 81: 2170–2176.
——— 2002. Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits. Proceedings of the Natural Academy of Sciences USA 99: 16823–16828.
Herrera, J. 1985. Nectar secretion patterns in southern Spanish Mediterranean shrublands. Annals of the Missouri Botanical Garden 74: 69–78.
——— 1992. Flower variation and breeding systems in the Cistaceae. Plant Systematics and Evolution 179: 245–255.
——— 2005. Flower size variation in Rosmarinus officinalis: individuals, populations and habitats. Annals of Botany 95: 431–437.
——— 2009. Visibility vs. biomass in flowers: exploring corolla allocation in Mediterranean entomophilous plants. Annals of Botany 103: 1119–1127.
Hodgins, K. A. & S. C. H. Barrett. 2008. Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus. Evolution 62: 1751–1763.
Irwin, R. E. 2006. The consequences of direct versus indirect species interactions to selection on traits: pollination and nectar robbing in Ipomopsis aggregata. American Naturalist 167: 315–328.
———, A. K. Brody & N. M. Waser. 2001. The impact of floral larceny on individuals, populations and communities. Oecologia 129: 161–168.
Joffre, R., S. Rambal & C. Damesin. 1999. Functional attributes in Mediterranean-type ecosystems. Pp 347–380. In: F. I. Pugnaire & F. Valladares (eds). Handbook of functional plant ecology. Marcel Dekker, Inc., New York, USA.
Johnson, S. G., L. F. Delph & C. L. Elderkin. 1995. The effect of petal-size manipulation on pollen removal, seed set, and insect-visitor behavior in Campanula americana. Oecologia 102: 174–179.
Jones, A. G. 2008. On the opportunity for sexual selection, the Bateman gradient and maximum intensity of sexual selection. Evolution 63: 1673–1684.
Jones, K. N. 2001. Pollinator-mediated assortative mating: causes and consequences. Pp 259–273. In: L. Chittka & J. D. Thomson (eds). Cognitive ecology of pollination: animal behaviour and floral evolution. Cambridge University Press, Cambridge, UK.
Lambers, H., F. S., III Chapin & T. L. Pons. 2008. Plant physiological ecology, ed. 2nd. Springer, New York.
Konsens, I., M. Ofir & J. Kigel. 1991. The effect of temperature on the production and abscission of flowers and pods in snap bean (Phaseolus vulgaris L.). Annals of Botany 67: 391–399.
Krupnick, G. A., A. E. Weis & D. R. Campbell. 1999. The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology 80: 125–134.
Kuriya, S., M. Hattori, Y. Nagano & T. Itino. 2015. Altitudinal flower size variation correlates with local pollinator size in a bumblebee-pollinated herb, Prunella vulgaris L. (Lamiaceae). Journal of Evolutionary Biology 28: 1761–1769.
Lambrecht, S. C. 2013. Flower water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae). International Journal of Plant Sciences 174: 74–84.
——— & T. E. Dawson. 2007. Correlated variation of floral and leaf traits along a moisture availability gradient. Oecologia 151: 574–583.
Larcher, W. 2000. Temperature stress and survival ability of Mediterranean schlerophyllous plants. Plant Biosystems 134: 279–295.
Lázaro, A., A. Jakobsson & Ø. Totland. 2013. How do pollinator visitation rate and seed set relate to species’ floral traits and community context? Oecologia 173: 881–893.
Liao, W.-J., Y. Hu, B.-R. Zhu, X.-Q. Zhao, Y.-F. Zeng & D.-Y. Zhang. 2009. Female reproductive success decreases with display size in monkshood, Aconitum kusnezoffii (Ranunculaceae). Annals of Botany 104: 1405–1412.
Maad, J. & R. Alexandersson. 2004. Variable selection in Platanthera bifolia (Orchidaceae): phenotypic selection differed between sex functions in a drought year. Journal of Evolutionary Biology 17: 642–650.
McCall, A. C. & R. E. Irwin. 2006. Florivory: the intersection of pollination and herbivory. Ecology Letters 9: 1351–1365.
Medel, R., A. Valiente, C. Botto-Mahan, G. Carvallo, F. Pérez, N. Pohl & L. Navarro. 2007. The influence of insects and hummingbirds on the geographical variation of the flower phenotype in Mimulus luteus. Ecography 30: 812–818.
Méndez, M. & A. Traveset. 2003. Sexual allocation in single-flowered hermaphroditic individuals in relation to plant and flower size. Oecologia 137: 69–75.
Mosleh Arany, A., T. J. de Jong & E. van der Meijden. 2009. Herbivory and local genetic differentiation in natural populations of Arabidopsis thaliana (Brassicaceae). Plant Ecology 201: 651–659.
Muñoz-Garmendía, F. & C. Navarro. 1993. Cistaceae. In: S. Castroviejo, C. Aedo, & M. Gómez-Campo (eds). Flora Iberica III: 318 − 436. CSIC, Madrid.
Nattero, J., R. Malerba, R. Medel & A. Cocucci. 2011. Factors affecting pollinator movement and plant fitness in a specialized pollination system. Plant Systematics and Evolution 296: 77–85.
Ninyerola, M., X. Pons & J.M. Roure. 2005. Atlas climático digital de la Península Ibérica [online]. UAB, Barcelona. http://opengis.uab.es/wms/iberia/espanol/es_cartografia.htm [accessed September 2011].
Nobel, P. S. & E. De la Barrera. 2000. Carbon and water balances for young fruits of platyopuntias. Physiologia Plantarum 109: 160–166.
Ohashi, K. & T. Yahara. 2001. Behavioral responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. Pp 274–296. In: L. Chittka & J. D. Thomson (eds). Cognitive ecology of pollination, animal behavior and floral evolution. Cambridge University Press, Cambridge, UK.
Oguro, M. & S. Sakai. 2015. Relation between flower head traits and florivory in Asteraceae: A phylogenetically controlled approach. American Journal of Botany 102: 407–416.
Olano, J. M., I. Caballero & A. Escudero. 2012. Soil seed bank recovery occurs more rapidly than expected in semi-arid Mediterranean gypsum vegetation. Annals of Botany 109: 299–307.
Patiño, S. & J. Grace. 2002. The cooling of convolvulaceous flowers in a tropical environment. Plant Cell and Environment 25: 41–51.
Petanidou, T., V. Goethals & E. Smets. 2000. Nectary structure of Labiatae in relation to their nectar secretion and characteristics in a Mediterranean shrub community—Does flowering time matter? Plant Systematics and Evolution 225: 103–118.
Potts, S. G., A. Dafni & G. Ne’eman. 2001. Pollination of a core flowering shrub species in Mediterranean phrygana: variation in pollinator diversity, abundance and effectiveness in response to fire. Oikos 92: 71–80.
Primack, R. B. 1985. Longevity of individual flowers. Annual Review of Ecology and Systematics 16: 15–37.
Pyke, G. H. 1991. What does it cost a plant to produce floral nectar? Nature 350: 58–59.
Quézel, P. & F. Médail. 2003. Ecologie et biogéographie des forêts du bassin méditerranéen. Elsevier, Paris.
Rhizopoulou, S., E. Ioannidi, N. Alexandredes & A. Argiropoulus. 2006. A study on functional and structural traits of the nocturnal flowers of Capparis spinosa L. Journal of Arid Environments 66: 635–647.
Rodriguez-Perez, J. 2005. Breeding system, flower visitors and seedling survival of two endangered species of Helianthemum (Cistaceae). Annals of Botany 95: 1229–1236.
Rosas‐Guerrero, V., R. Aguilar, S. Martén‐Rodríguez, L. Ashworth, M. Lopezaraiza‐Mikel, J. M. Bastida & M. Quesada. 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecology Letters 17: 388–400.
Ross, J. D. & C. Sombrero. 1991. Pp 83–94. Environmental control of essential oil production in Mediterranean plants. Ecological chemistry and biochemistry of plant terpenoids. Clarendon, Oxford.
Ruane, L. G., A. T. Rotzin & P. H. Congleton. 2014. Floral display size, conspecific density and florivory affect fruit set in natural populations of Phlox hirsuta, an endangered species. Annals of Botany 113: 887–893.
Sahli, H. F. & J. K. Conner. 2011. Testing for conflicting and nonadditive selection: floral adaptation to multiple pollinators through male and female fitness. Evolution 65: 1457–1473.
Schemske, D. W. & C. Horvitz. 1988. Plant-animal interactions and fruit production in a neotropical herb: a path analysis. Ecology 69: 1128–1137.
Seufert, G., D. Kotzias, C. Sparta & B. Versino. 1995. Volatile organics in Mediterranean shrubs and their potential role in a changing environment. In Global change and Mediterranean-type ecosystems (pp. 343 − 370). Springer New York.
Shykoff, J. A., E. Bucheli & O. Kaltz. 1996. Flower lifespan and disease risk. Nature 379: 779–780.
Smith, S. D., C. Ané & D. A. Baum. 2008. The role of pollinator shifts in the floral diversification of Iochroma (Solanaceae). Evolution 62: 793–806.
Snow, A. A. & P. O. Lewis. 1993. Reproductive traits and male fertility in plants: empirical approaches. Annual Review of Ecology and Systematics 24: 331–351.
Sprengel, C. K. 1793. Das entdeckte geheimnis der natur im bau und in der befruchtung der blumen. Friedrich Vieweg, Berlin, Germany.
Stanton, M. L., A. A. Snow & S. N. Handel. 1986. Floral evolution: attractiveness to pollinators increases male fitness. Science 232: 1625–1627.
Stebbins, G. L. 1950. Variation and evolution in plants. Columbia University Press, New York, USA.
——— 1970. Adaptive radiation of reproductive characteristics in angiosperms: I. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326.
Strauss, S. Y. & J. B. Whittall. 2006. Non-pollinator agents of selection on floral traits. Pp 120–138. In: L. D. Harder & S. C. H. Barrett (eds). Ecology and evolution of flowers. Oxford University Press, Oxford, UK.
Talavera, S., F. Bastida, P. L. Ortiz & M. Arista. 2001. Pollinator attendance and reproductive success in Cistus libanotis L. (Cistaceae). International Journal of Plant Sciences 162: 343–352.
———, P. E. Gibbs & M. Arista. 1997. Reproductive biology of Halimium atriplicifolium (Lam.) Spach and H. halimifolium (L.) Willk. (Cistaceae). Lagascalia 19: 571–578.
———, ——— & J. Herrera. 1993. Reproductive biology of Cistus ladanifer (Cistaceae). Plant Systematics and Evolution 186: 123–134.
Teixido, A. L. 2014. Indirect costs counteract the effects of pollinator-mediated phenotypic selection on corolla size in the Mediterranean shrub Halimium atriplicifolium. Journal of Plant Ecologyogy 7: 364–372.
——— & F. Valladares. 2013. Large and abundant flowers increase indirect costs of corollas: a study of coflowering sympatric Mediterranean species of contrasting flower size. Oecologia 173: 73–81.
——— & ———. 2014a. Disproportionate carbon and water maintenance costs of large corollas in hot Mediterranean ecosystems. Perspectives in Plant Ecologyogy, Evolution and Systematics 16: 83–92.
——— & ———. 2014b. Pollinator-mediated phenotypic selection does not always modulate flower size and number in the large-flowered Mediterranean shrub Cistus ladanifer (Cistaceae). Botanical Journal of the Linnean Society 176: 540–555.
——— & ———. 2014c. Large flowers tend to be short-lived in Mediterranean ecosystems: insights from three Cistus species. Plant Biosystems 148: 1211–1220.
——— & ———. 2015. Temperature-limited floral longevity in the large-flowered Mediterranean shrubCistus ladanifer (Cistaceae). International Journal of Plant Sciences 176: 131–140.
———, M. Méndez & F. Valladares. 2011. Flower size and longevity influence florivory in the large-flowered shrub Cistus ladanifer. Acta Oecologica 37: 418–421.
Thompson, J. D. 2001. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126: 386–394.
——— 2005. Plant evolution in the Mediterranean. Oxford University Press, New York.
Totland, Ø. 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82: 2233–2244.
Ushimaru, A., S. Kikuchi, R. Yonekura, A. Maruyama, N. Yanagisawa, M. Kagami, M. Nakagawa, S. Mahoro, Y. Kohmatsu, A. Hatada, S. Kitamura & K. Nakata. 2006. The influence of floral symmetry and pollination systems on flower size variation. Nordic Journal of Botany 24: 593–598.
Valiente-Banuet, A., A. Rojas-Martínez, A. Casas, M. C. Arizmendi & P. Dávila. 1997. Pollination biology of two winter-blooming giant columnar cacti in the Tehuacán Valley, México. Journal of Arid Environments 37: 1–11.
Valladares, F., E. Martínez-Ferri, L. Balaguer, E. Pérez-Corona & E. Manrique. 2000. Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource-use strategy? New Phytologist 148: 79–91.
———, A. Vilagrosa, J. Peñuelas, R. Ogaya, J.J. Camarero, L. Corcuera, S. Sisó & E. Gil-Pelegrín. 2004. Estrés hídrico, ecofisiología y escalas de la sequía. Pp. 163 − 190. In: F. Valladares (ed.), Ecología del bosque mediterráneo en un mundo cambiante. Ministerio de Medio Ambiente, EGRAF, S.A, Madrid, Spain.
———, J. Zaragoza-Castells, D. Sánchez-Gómez, S. Matesanz, B. Alonso, A. Portsmouth, A. Delgado & O. K. Atkin. 2008. Is shade beneficial for Mediterranean shrubs experiencing periods of extreme drought and late-winter frosts? Annals of Botany 102: 923–933.
Vemmos, S. N. & G. K. Goldwin. 1994. The photosynthetic activity of Cox’s orange pippin apple flowers in relation to fruit setting. Annals of Botany 73: 385–391.
Verdú, M., J. Barrón-Sevilla, A. Valiente-Banuet, N. Flores-Hernández & P. García-Fayos. 2002. Mexical plant phenology: is it similar to mediterranean communities? Botanical Journal of the Linnean Society 138: 297–303.
Wade, M. J. 1979. Sexual selection and variance in reproductive success. American Naturalist 114: 742–747.
Willmer, P. 2011. Pollination and floral ecology. Princeton University Press, Princeton, NJ, USA.
Witt, T., A. Jürgens & G. Gottsberger. 2013. Nectar sugar composition of European Caryophylloideae (Caryophyllaceae) in relation to flower length, pollination biology and phylogeny. Journal of Evolutionary Biology 26: 2244–2259.
Young, H. J. & M. L. Stanton. 1990. Influences of floral variation on pollen removal and seed production in wild radish. Ecology 71: 536–547.
Zaragoza‐Castells, J., D. Sánchez‐Gómez, I. P. Hartley, S. Matesanz, F. Valladares, J. Lloyd & O. K. Atkin. 2008. Climate‐dependent variations in leaf respiration in a dry‐land, low productivity Mediterranean forest: the importance of acclimation in both high‐light and shaded habitats. Functional Ecology 22: 172–184.
Acknowledgments
We thank A. Escudero, J.M. Iriondo, J. Arroyo, P. García-Fayos, A. Sánchez, A. Traveset, S. Karrenberg, J. Ågren, N. Sletvold, A.L. Parachnowitsch, C.M. Caruso, J. Herrera, J. Ollerton, J.F. Scheepens, J. Těšitel and M.A. Rodríguez-Gironés for the comments provided during the first versions of the manuscript. We are also grateful to Y. Valiñani and E. Galisteo for lab assistance and to J.P. González-Varo, J. Güemes, E. Carrió, E. Triano and R. Torices for collecting flower buds for analyses of floral production costs. J. Herrera kindly provided some data of flower size for several Mediterranean species.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixido, A.L., Barrio, M. & Valladares, F. Size Matters: Understanding the Conflict Faced by Large Flowers in Mediterranean Environments. Bot. Rev. 82, 204–228 (2016). https://doi.org/10.1007/s12229-016-9168-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-016-9168-8