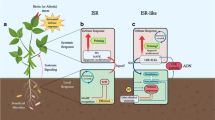
Abstract
Rhizobia are a group of organisms that are well known for their ability to colonize root surfaces and form symbiotic associations with legume plants. They not only play a major role in biological nitrogen fixation but also improve plant growth and reduce disease incidence in various crops. Rhizobia are known to control the growth of many soilborne plant pathogenic fungi belonging to different genera like Fusarium, Rhizoctonia, Sclerotium, and Macrophomina. Antagonistic activity of rhizobia is mainly attributed to production of antibiotics, hydrocyanic acid (HCN), mycolytic enzymes, and siderophore under iron limiting conditions. Rhizobia are also reported to induce systemic resistance and enhance expression of plant defense-related genes, which effectively immunize the plants against pathogens. Seed bacterization with appropriate rhizobial strain leads to elicitation and accumulation of phenolic compounds, isoflavonoid phytoalexins, and activation of enzymes like L-phenylalanine ammonia lyase (PAL), chalcone synthase (CHS), peroxidase (POX), polyphenol oxidase (PPO), and others involved in phenylpropanoid and isoflavonoid pathways. Development of Rhizobium inoculants with dual attributes of nitrogen fixation and antagonism against phytopathogens can contribute to increased plant growth and productivity. This compilation aims to bring together the available information on the biocontrol facet of rhizobia and identify research gaps and effective strategies for future research in this area.


Similar content being viewed by others
References
Akhtar MS, Siddiqui ZA (2008) Biocontrol of a root-rot disease complex of chickpea by Glomus intraradices, Rhizobium sp. and Pseudomonas straita. Crop Prot 27:410–417
Akibode S, Maredia M (2011) Global and regional trends in production, trade and consumption of food legume crops. CGIAR Draft Report submitted to the Standing Panel on Impact Assessment (SPIA) of the CGIAR Science Council, FAO, Rome, March 2011. Available: http://impact.cgiar.org/sites/default/files/images/Legumetrendsv2.pdf
Al-Ani RA, Adhab MA, Mahdi MH, Abood HM (2012) Rhizobium japonicum as a biocontrol agent of soybean root rot disease caused by Fusarium solani and Macrophomina phaseolina. Plant Prot Sci 48:149–155
Albareda M, Rodríguez-Navarro DN, Camacho M, Temprano FJ (2008) Alternatives to peat as a carrier for rhizobia inoculants: solid and liquid formulations. Soil Biol Biochem 40:2771–2779. doi:10.1016/j.soilbio.2008.07.021
Antoun H, Bordeleau LM, Gagnon C (1978) Antagonisme entre Rhizobium meliloti at Fusarium oxysporum en relation avec lefficacite symbiotique. Can J Plant Sci 58:75–78. doi:10.4141/cjps78-014
Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.) Plant Soil 204:57–67. doi:10.1023/a:1004326910584
Arfaoui A, Sifi B, El Hassni M, El Hadrami I, Boudabbous A, Chérif M (2005) Biochemical analysis of chickpea protection against Fusarium wilt afforded by two Rhizobium isolates. Plant Pathol J 4:35–42
Arfaoui A, Sifi B, Boudabous A, Hadrami IE, Chérif M (2006) Identification of Rhizobium isolates possessing antagonistic activity against Fusarium oxysporum f. sp. ciceris, the causal agent of Fusarium wilt of chickpea. J Plant Pathol 88:67–75
Arfaoui A, El Hadrami A, Mabrouk Y, Sifi B, Boudabous A, El Hadrami I, Daayf F, Cherif M (2007) Treatment of chickpea with Rhizobium isolates enhances the expression of phenylpropanoid defense-related genes in response to infection by Fusarium oxysporum f. sp. ciceris. Plant Physiol Biochem 45:470–479. doi:10.1016/j.plaphy.2007.04.004
Arora N, Kang S, Maheshwari D (2001) Isolation of siderophore producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci 81:673–677
Babu S, Prasanna R, Bidyarani N, Nain L, Shivay YS (2015) Synergistic action of PGP agents and Rhizobium spp. for improved plant growth, nutrient mobilization and yields in different leguminous crops. Biocatal Agric Biotechnol 4:456–464. doi:10.1016/j.bcab.2015.09.004
Baddeley JA, Jones S, Topp CFE, Watson CA, Helming J, Stoddard FL (2014) Biological nitrogen fixation (BNF) by legume crops in Europe. In: Legume Futures Report 1.5. Legume-supported cropping systems for Europe. Available online at: www.legumefutures.eu
Balasundaran V, Sarbhoy A (1988) Inhibition of plant pathogenic fungi by Rhizobium japonicum. Indian Phytopathol 41:128–130
Bardin SD, Huang H-C, Pinto J, Amundsen EJ, Erickson RS (2004) Biological control of Pythium damping-off of pea and sugar beet by Rhizobium leguminosarum bv. Viceae. Can J Bot 82:291–296. doi:10.1139/b04-003
Bashan Y, Levanony H (1990) Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can J Microbiol 36:591–608. doi:10.1139/m90-105
Beauchamp CJ, Dion P, Kloepper JW, Antoun H (1991) Physiological characterization of opine-utilizing rhizobacteria for traits related to plant growth-promoting activity. Plant Soil 132:273–279. doi:10.1007/bf00010408
Beijerinck MW (1888) Die Bacterian der Papillionaceenknollchen. Bot Ztg 46:726–725-804
Bidyarani N, Prasanna R, Babu S, Hossain F, Saxena AK (2016) Enhancement of plant growth and yields in chickpea (Cicer arietinum L.) through novel cyanobacterial and biofilmed inoculants. Microbiol Res. doi:10.1016/j.micres.2016.04.005
Bohlool BB, Ladha JK, Garrity DP, George T (1992) Biological nitrogen fixation for sustainable agriculture: a perspective. Plant Soil 141:1–11. doi:10.1007/bf00011307
Brockwell J, Bottomley PJ (1995) Recent advances in inoculant technology and prospects for the future. Soil Biol Biochem 27:683–697. doi:10.1016/0038-0717(95)98649-9
Buonassisi AJ, Copeman RJ, Pepin HS, Eaton GW (1986) Effect of Rhizobium spp. on Fusarium solani f. sp. phaseoli. Can J Plant Pathol 8:140–146. doi:10.1080/07060668609501817
Burr T, Schroth M, Suslow T (1978) Increased potato yields by treatment of seedpieces with specific strains of Pseudomonas fluorescens and Pseudomonas putida [bacterization]. Phytopathology 68:1377–1383
Carson KC, Meyer J-M, Dilworth MJ (2000) Hydroxamate siderophores of root nodule bacteria. Soil Biol Biochem 32:11–21. doi:10.1016/S0038-0717(99)00107-8
Chakraborty U, Chakraborty BN (1989) Interaction of Rhizobium leguminosarum and Fusarium solani f. sp. pisi on pea affecting disease development and phytoalexin production. Can J Bot 67:1698–1701. doi:10.1139/b89-214
Chakraborty U, Purkayastha RP (1984) Role of rhizobitoxine in protecting soybean roots from Macrophomina phaseolina infection. Can J Microbiol 30:285–289. doi:10.1139/m84-043
Chand H, Khirbat S (2009) Chickpea wilt and its management—a review. Agricul Rev 30:1–12
Chandra S, Choure K, Dubey RC, Maheshwari DK (2007) Rhizosphere competent Mesorhizobium loti MP6 induces root hair curling, inhibits Sclerotinia sclerotiorum and enhances growth of Indian mustard (Brassica campestris). Braz J Microbiol 38:124–130
Chao WL (1990) Antagonistic activity of Rhizobium spp. against beneficial and plant pathogenic fungi. Lett Appl Microbiol 10:213–215. doi:10.1111/j.1472-765X.1990.tb01336.x
Chiarini L, Bevivino A, Tabacchioni S, Dalmastri C (1998) Inoculation of Burkholderia cepacia, Pseudomonas fluorescens, and Enterobacter sp. on Sorghum bicolor: root colonization and plant growth promotion of dual strain inocula. Soil Biol Biochem 30:81–87. doi:10.1016/S0038-0717(97)00096-5
Crowley DE (2006) Microbial siderophores in the plant rhizosphere. In: Barton LL, Abadia J (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer Netherlands, Dordrecht, pp 169–198. doi:10.1007/1-4020-4743-6_8
Crowley DE, Gries D (1994) Modeling of iron availability in the plant rhizosphere. In: Biochemistry of metal micronutrients in the rhizosphere. Lewis Publishers, Boca Raton, Florida, pp 199–224
Dakora FD (2003) Defining new roles for plant and rhizobial molecules in sole and mixed plant cultures involving symbiotic legumes. New Phytol 158:39–49
Date R, Roughley R (1977) Preparation of legume seed inoculants a treatise on dinitrogen fixation section IV agronomy and ecology. Wiley, New York, pp 243–275
Denardin ND, Freire JRJ (2000) Assessment of polymers for the formulation of legume inoculants. World J Microbiol Biotechno 16:215–217. doi:10.1023/a:1008914223467
Deshwal V, Dubey R, Maheshwari D (2003a) Isolation of plant growth-promoting strains of Bradyrhizobium (Arachis) sp. with biocontrol potential against Macrophomina phaseolina causing charcoal rot of peanut. Curr Sci 84:443–448
Deshwal V, Pandey P, Kang S, Maheshwari D (2003b) Rhizobia as a biological control agent against soil borne plant pathogenic fungi. Indian J Exp Biol 41:1160–1164
Dilworth MJ, Carson KC, Giles RGF, Byrne L, Glenn AR (1998) Rhizobium leguminosarum bv. viciae produces a novel cyclic trihydroxamate siderophore, vicibactin. Microbiology 144:781–791
Dutta S, Mishra A, Kumar BD (2008) Induction of systemic resistance against fusarial wilt in pigeon pea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol Biochem 40:452–461
Ehteshamul-Haque S, Ghaffar A (1993) Use of rhizobia in the control of root rot diseases of sunflower, okra, soybean and mungbean. J Phytopathol 138:157–163
Elkan GH (1992) Taxonomy of the rhizobia. Can J Microbiol 38:446–450. doi:10.1139/m92-075
Essalmani H, Lahlou H (2002) In vitro antagonistic activity of some microorganisms towards Fusarium oxysporum f. sp. lentis (french). Crypto Mycol 23:221–234
Estevez de Jensen C, Percich JA, Graham PH (2002) Integrated management strategies of bean root rot with Bacillus subtilis and Rhizobium in Minnesota. Field Crops Res 74:107–115. doi:10.1016/S0378-4290(01)00200-3
Fernandes Júnior PI, Rohr TG, Oliveira PTD, Xavier GR, Rumjanek NG (2009) Polymers as carriers for rhizobial inoculant formulations. Pesq Agrop Brasileira 44:1184–1190
Frank B (1889) Uber die Pilzsymbiose der Leguminosen. Berichte der Deutschen Botanischen Gesellschaft 7:332–346
Ganesan S, Kuppusamy RG, Sekar R (2007) Integrated management of stem rot disease (Sclerotium rolfsii) of groundnut (Arachis hypogaea L.) using Rhizobium and Trichoderma harzianum (ITCC-4572). Turk J Agric For 31:103–108
Gao X, Lu X, Wu M, Zang H, Pan R, Tian J, Li S, Liao H (2012) Co-inoculation with rhizobia and AMF inhibited soybean red crown rot: from field study to plant defense-related gene expression analysis. PLoS One 7:e33977
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CLL, Krishnamurthy L (2015) Plant growth promoting rhizobia: challenges and opportunities. 3. Biotech 5:355–377. doi:10.1007/s13205-014-0241-x
Guerinot ML, Meidl EJ, Plessner O (1990) Citrate as a siderophore in Bradyrhizobium japonicum. J Bacteriol 172:3298–3303
Hafeez FY, Naeem FI, Naeem R, Zaidi AH, Malik KA (2005) Symbiotic effectiveness and bacteriocin production by Rhizobium leguminosarum bv. viciae isolated from agriculture soils in Faisalabad. Environ Exp Bot 54:142–147. doi:10.1016/j.envexpbot.2004.06.008
Hellriegel H, Wilfarth H (1888) Untersuchungenüber die stickstoffnahrung der Gramineen und Leguminosen. Belageheftc zu der zeitschrift des vereinsc Rübenzucker-Industrie Deutschen, Reiches 1:234
Hemissi I, Mabrouk Y, Abdi N, Bouraoui M, Saidi M, Sifi B (2011) Effects of some Rhizobium strains on chickpea growth and biological control of Rhizoctonia solani. Afr J Microbiol Res 5:4080–4090
Herridge DF, Peoples MB, Boddey RM (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18. doi:10.1007/s11104-008-9668-3
Hirsch PR (1979) Plasmid-determined bacteriocin production by Rhizobium leguminosarum. Microbiology 113:219–228. doi:10.1099/00221287-113-2-219
Hirsch PR, Van Montagu M, Johnston AWB, Brewin NJ, Schell J (1980) Physical identification of bacteriocinogenic, nodulation and other plasmids in strains of Rhizobium leguminosarum. Microbiology 120:403–412. doi:10.1099/00221287-120-2-403
Huang HC, Erickson RS (2007) Effect of seed treatment with Rhizobium leguminosarum on Pythium damping-off, seedling height, root nodulation, root biomass, shoot biomass, and seed yield of pea and lentil. J Phytopathol 155:31–37. doi:10.1111/j.1439-0434.2006.01189.x
Ivanova E, Teunou E, Poncelet D(2005) Alginate-based macrocapsules as inoculants carriers for production of nitrogen biofertilizers. In: Proceedings of the Balkan Scientific Conference of Biology, Plvdiv, Bulgaria (Eds. B Gruev, M Nikolova, A Donev). pp 90–108
Janczarek M, Rachwał K, Marzec A, Grządziel J, Palusińska-Szysz M (2015) Signal molecules and cell-surface components involved in early stages of the legume–rhizobium interactions. Appl Soil Ecol 85:94–113. doi:10.1016/j.apsoil.2014.08.010
Jayasinghearachchi HS, Seneviratne G (2004) A bradyrhizobial-Penicillium spp. biofilm with nitrogenase activity improves N2 fixing symbiosis of soybean. Biol Fert Soils 40:432–434. doi:10.1007/s00374-004-0796-5
Jia RZ, Zhang RJ, Wei Q, Chen WF, Cho IK, Chen WX, Li QX (2015) Identification and classification of rhizobia by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Proteomics Bioinform 8:98–107. doi:10.4172/jpb.1000357
Jordan D (1984) Family III. In: Rhizobiaceae Bergey’s manual of systematic bacteriology, vol 1, pp 234–242
Kelemu S, Thomas RJ, Moreno CX, Ocampo GI (1995) Strains of Bradyrhizobium from tropical forage legumes inhibit Rhizoctonia solani AG-1 in vitro. Australas Plant Pathol 24:168–172
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70. doi:10.1016/j.cropro.2012.01.007
Krishnan HB, Kang BR, Hari Krishnan A, Kim KY, Kim YC (2007) Rhizobium etli USDA9032 engineered to produce a phenazine antibiotic inhibits the growth of fungal pathogens but is impaired in symbiotic performance. Appl Environ Microbiol 73:327–330. doi:10.1128/aem.02027-06
Kumar Singh A, Prasad Bhatt R, Pant S (2012) Comparative study of carrier based materials for Rhizobium culture formulation. Indian J Agric Res 46:344–349
Kumar H, Bajpai VK, Dubey RC, Maheshwari DK, Kang SC (2010) Wilt disease management and enhancement of growth and yield of Cajanus cajan (L) var. Manak by bacterial combinations amended with chemical fertilizer. Crop Prot 29:591–598. doi:10.1016/j.cropro.2010.01.002
Kumar H, Dubey RC, Maheshwari DK (2011) Effect of plant growth promoting rhizobia on seed germination, growth promotion and suppression of Fusarium wilt of fenugreek (Trigonella foenum-graecum L.) Crop Prot 30:1396–1403. doi:10.1016/j.cropro.2011.05.001
Ligon JM, Hill DS, Hammer PE, Torkewitz NR, Hofmann D, Kempf HJ, Pée KHV (2000) Natural products with antifungal activity from Pseudomonas biocontrol bacteria. Pest Manag Sci 56:688–695
Lupwayi N, Olsen P, Sande E, Keyser H, Collins M, Singleton P, Rice W (2000) Inoculant quality and its evaluation. Field Crops Res 65:259–270
Mabrouk Y, Zourgui L, Sifi B, Delavault P, Simier P, Belhadj O (2007) Some compatible Rhizobium leguminosarum strains in peas decrease infections when parasitised by Orobanche crenata. Weed Res 47:44–53. doi:10.1111/j.1365-3180.2007.00548.x
Malajczuk N, Pearce M, Litchfield RT (1984) Interactions between Phytophthora cinnamomi and Rhizobium isolates. Trans Br Mycol Soc 82:491–500. doi:10.1016/S0007-1536(84)80014-5
Malusá E, Sas-Paszt L, Ciesielska J (2012) Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J 2012:491206. doi:10.1100/2012/491206
Martínez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML (2010) Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr 10:293–319
Matzanke BF (1991) Structures, coordination chemistry and functions of microbial iron chelates. In: Winkelmann G (ed) Handbook of microbial iron chelates. CRC Press, Boca Raton, Fla, pp 15–64
Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS (2001) Functional analysis of genes for biosynthesis of Pyocyanin and phenazine-1-Carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183:6454–6465. doi:10.1128/jb.183.21.6454-6465.2001
Mazen M, El-Batanony NH, Abd El-Monium M, Massoud O (2008) Cultural filtrate of Rhizobium spp. and arbuscular mycorrhiza are potential biological control agents against root rot fungal diseases of faba bean. Global J Biotechnol Biochem 3:32–41
Mazur S, Nawrocki J, Kućmierz J (2004) Disease symptoms on chickpea (Cicer arietinum L.) and their causal agents. Folia Hortic 16:47–53
Mishra RP, Singh RK, Jaiswal HK, Kumar V, Maurya S (2006) Rhizobium-mediated induction of phenolics and plant growth promotion in rice (Oryza sativa L.) Curr Microbiol 52:383–389
Modi M, Shah KS, Modi VV (1985) Isolation and characterisation of catechol-like siderophore from cowpea Rhizobium RA-1. Arch Microbiol 141:156–158. doi:10.1007/bf00423277
Moulin L, Munive A, Dreyfus B, Boivin-Masson C (2001) Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature 411:948–950. doi:10.1038/35082070
Nadia H, Massoud O, Mazen M, El-Monium MA (2007) The inhibitory effects of cultural filtrates of some wild Rhizobium spp. on some faba bean root rot pathogens and their antimicrobial synergetic effect when combined with Arbusclar mycorrhiza (AM). World J Agric Sci 3:721–730
Nautiyal CS (1997) Rhizosphere competence of Pseudomonas sp. NBRI9926 and Rhizobium sp. NBRI9513 involved in the suppression of chickpea (Cicer arietinum L.) pathogenic fungi. FEMS Microbiol Ecol 23:145–158. doi:10.1111/j.1574-6941.1997.tb00398.x
Nobbe F, Hiltner L (1896) Inoculation of the soil for cultivating leguminous plants. US patent 570:813
Omar SA, Abd-Alla MH (1998) Biocontrol of fungal root rot diseases of crop plants by the use of rhizobia and bradyrhizobia. Folia Microbiol 43:431–437. doi:10.1007/bf02818587
Osdaghi E, Shams-Bakhsh M, Alizadeh A, Lak MR, Maleki HH (2011) Induction of resistance in common bean by Rhizobium leguminosarum bv. phaseoli and decrease of common bacterial blight. Phytopathol Mediterr 50:45–54
Ozkoc I, Deliveli MH (2001) In vitro inhibition of the mycelial growth of some root rot fungi by Rhizobium leguminosarum biovar phaseoli isolates. Turk J Biol 25:435–445
Patel HN, Chakraborty RN, Desai SB (1988) Isolation and partial characterization of phenolate siderophore from Rhizobium leguminosarum IARI 102. FEMS Microbiol Lett 56:131–134
Peoples MB, Craswell ET (1992) Biological nitrogen fixation: investments, expectations and actual contributions to agriculture. Plant Soil 141:13–39. doi:10.1007/bf00011308
Persmark M, Pittman P, Buyer JS, Schwyn B, Gill PR, Neilands JB (1993) Isolation and structure of rhizobactin 1021, a siderophore from the alfalfa symbiont Rhizobium meliloti 1021. J Am Chem Soc 115:3950–3956. doi:10.1021/ja00063a014
Phillips DA, Kapulnik Y (1995) Plant isoflavonoids, pathogens and symbionts. Trends Microbiol 3:58–64
Prasanna R, Kumar A, Babu S, Chawla G, Chaudhary V, Singh S, Gupta V, Nain L, Saxena AK (2013) Deciphering the biochemical spectrum of novel cyanobacterium-based biofilms for use as inoculants. Biol Agric Hortic 29:145–158. doi:10.1080/01448765.2013.790303
Prasanna R, Triveni S, Bidyarani N, Babu S, Yadav K, Adak A, Khetarpal S, Pal M, Shivay YS, Saxena AK (2014) Evaluating the efficacy of cyanobacterial formulations and biofilmed inoculants for leguminous crops. Arch Agron Soil Sci 60:349–366
Prasanna R, Adak A, Verma S, Bidyarani N, Babu S, Pal M, Shivay YS, Lata N (2015) Cyanobacterial inoculation in rice grown under flooded and SRI modes of cultivation elicits differential effects on plant growth and nutrient dynamics. Ecol Engg 84:532–541
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Anton van Leeuwen 81:537–547. doi:10.1023/a:1020501420831
Rabie GH (1998) Induction of fungal disease resistance in Vicia faba by dual inoculation with Rhizobium leguminosarum and vesicular-arbuscular mycorrhizal fungi. Mycopathologia 141:159–166. doi:10.1023/a:1006937821777
Ramos T, Bellaj ME, Idrissi-Tourane AE, Daayf F, Hadrami IE (1997) Les Phénolamides des Rachis de Palmes, Composants de la Réaction de Défense du Palmier Dattier vis-à-vis de Fusarium oxysporum f.sp. albedinis, Agent Causal du Bayoud. J Phytopathol 145:487–493. doi:10.1111/j.1439-0434.1997.tb00355.x
Rebah FB, Prévost D, Yezza A, Tyagi R (2007) Agro-industrial waste materials and wastewater sludge for rhizobial inoculant production: a review. Bioresour Technol 98:3535–3546
Reitz M, Rudolph K, Schröder I, Hoffmann-Hergarten S, Hallmann J, Sikora RA (2000) Lipopolysaccharides of Rhizobium etli strain G12 act in potato roots as an inducing agent of systemic resistance to infection by the cyst nematode Globodera pallida. Appl Env Microbiol 66:3515–3518
Rioux CR, Jordan DC, Rattray JBM (1986) Iron requirement of Rhizobium leguminosarum and secretion of anthranilic acid during growth on an iron-deficient medium. Arch Biochem Biophys 248:175–182. doi:10.1016/0003-9861(86)90414-5
Robleto EA, Borneman J, Triplett EW (1998) Effects of bacterial antibiotic production on rhizosphere microbial communities from a culture-independent perspective. Appl Env Microbiol 64:5020–5022
Roughley RJ, Vincent J (1967) Growth and survival of Rhizobium spp. in peat culture. J Appl Bacteriol 30:362–376
Ryan PR, Dessaux Y, Thomashow LS, Weller DM (2009) Rhizosphere engineering and management for sustainable agriculture. Plant Soil 321:363–383. doi:10.1007/s11104-009-0001-6
Schwinghamer EA, Brockwell J (1978) Competitive advantage of bacteriocin and phage-producing strains of Rhizobium trifolii in mixed culture. Soil Biol Biochem 10:383–387. doi:10.1016/0038-0717(78)90062-7
Seneviratne G (2003) Development of eco-friendly, beneficial microbial biofilms. Curr Sci 85:1395–1396
Seneviratne G, Zavahir JS, Bandara WMMS, Weerasekara MLMAW (2008) Fungal-bacterial biofilms: their development for novel biotechnological applications. World J Microbiol Biotechnol 24:739–743. doi:10.1007/s11274-007-9539-8
Shaban W, El-Bramawy M (2011) Impact of dual inoculation with Rhizobium and Trichoderma on damping off, root rot diseases and plant growth parameters of some legumes field crop under greenhouse conditions. Intl Res J Agric Sci Soil Sci 1:98–108
Sharif T, Khalil S, Ahmad S (2003) Effect of Rhizobium sp. on growth of pathogenic fungi under in vitro conditions. Pakistan J Biol Sci 6:1597–1599
Siddiqui ZA (2006) PGPR: prospective biocontrol agents of plant pathogens. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer Netherlands, Dordrecht, pp 111–142. doi:10.1007/1-4020-4152-7_4
Siddiqui IA, Shaukat SS (2002) Mixtures of plant disease suppressive bacteria enhance biological control of multiple tomato pathogens. Biol Fert Soils 36:260–268
Siddiqui IA, Ehteshamul-Haque S, Zaki MJ, Abdul G (2000) Effect of urea on the efficacy of Bradyrhizobium sp. and Trichoderma harzianum in the control of root infecting fungi in mungbean and sunflower. Sarhad J Agric 16:403–406
Singh PK, Singh M, Vyas D (2010) Biocontrol of fusarium wilt of chickpea using arbuscular mycorrhizal fungi and Rhizobium leguminosorum biovar. Caryologia 63:349–353
Sitrit Y, Barak Z, Kapulnik Y, Oppenheim A, Chet I (1993) Expression of Serratia marcescens chitinase gene in Rhizobium meliloti during symbiosis on alfalfa roots. Mol Plant-Microbe Interact 6:293–298
Smith RS (1992) Legume inoculant formulation and application. Can J Microbiol 38:485–492. doi:10.1139/m92-080
Smith MJ, Shoolery JN, Schwyn B, Holden I, Neilands JB (1985) Rhizobactin, a structurally novel siderophore from Rhizobium meliloti. J Am Chem Soc 107:1739–1743. doi:10.1021/ja00292a047
Smitha M, Singh R (2014) Biocontrol of phytopathogenic fungi using mycolytic enzymes produced by rhizospheric bacteria of Cicer arietinum. Indian J Agric Biochem 27:215–218
Sridevi M, Mallaiah K (2008) Factors effecting chitinase activity of Rhizobium sp. from Sesbania sesban. Biologia 63:307–312
Stephens JHG, Rask HM (2000) Inoculant production and formulation. Field Crops Res 65:249–258. doi:10.1016/S0378-4290(99)00090-8
Temprano F, Albareda M, Camacho M, Daza A, Santamaría C, Rodríguez-Navarro ND (2002) Survival of several Rhizobium/Bradyrhizobium strains on different inoculant formulations and inoculated seeds. Int Microbiol 5:81–86. doi:10.1007/s10123-002-0067-y
Tittabutr P, Payakapong W, Teaumroong N, Singleton PW, Boonkerd N (2007) Growth, survival and field performance of bradyrhizobial liquid inoculant formulations with polymeric additives. Sci Asia 33:69–77
Triplett EW, Barta TM (1987) Trifolitoxin production and nodulation are necessary for the expression of superior nodulation competitiveness by Rhizobium leguminosarum bv. trifolii strain T24 on clover. Plant Physiol 85:335–342
Triveni S, Prasanna R, Shukla L, Saxena AK (2013) Evaluating the biochemical traits of novel Trichoderma-based biofilms for use as plant growth-promoting inoculants. Ann Microbiol 63:1147–1156. doi:10.1007/s13213-012-0573-x
Triveni S, Prasanna R, Kumar A, Bidyarani N, Singh R, Saxena AK (2015) Evaluating the promise of Trichoderma and Anabaena based biofilms as multifunctional agents in Macrophomina phaseolina-infected cotton crop. Biocont Sci Technol 25:656–670. doi:10.1080/09583157.2015.1006171
Tu JC (1978) Protection of soybean from severe Phytophthora root rot by Rhizobium. Physiol Plant Pathol 12:233–240. doi:10.1016/0048-4059(78)90065-6
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397
Vandergheynst J, Scher H, Guo H-Y, Schultz D (2007) Water-in-oil emulsions that improve the storage and delivery of the biolarvacide Lagenidium giganteum. BioControl 52:207–229. doi:10.1007/s10526-006-9021-9
Weidemann C, Tenhaken R, Höhl U, Barz W (1991) Medicarpin and maackiain 3-O-glucoside-6′-O-malonate conjugates are constitutive compounds in chickpea (Cicer arietinum L.) cell cultures. Plant Cell Rep 10:371–374. doi:10.1007/bf00193162
Weigand F, Köster J, Weltzien H, Barz W (1986) Accumulation of phytoalexins and isoflavone glucosides in a resistant and a susceptible cultivar of Cicer arietinum during infection with Ascochyta rabiei. J Phytopathol 115:214–221
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407. doi:10.1146/annurev.py.26.090188.002115
Weller DM, Cook RJ (1986) Suppression of root diseases of wheat by fluorescent pseudomonads and mechanisms of action. In: Swinburne TR (ed) Iron, siderophores, and plant diseases. Springer US, Boston, MA, pp 99–107. doi:10.1007/978-1-4615-9480-2_12
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin a by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963. doi:10.1016/S0038-0717(02)00027-5
Zakhia F, De Lajudie P (2001) Taxonomy of rhizobia. Agronomie 21:569–576. doi:10.1051/agro:2001146
Acknowledgements
The authors are thankful to the Council of Scientific and Industrial Research (CSIR), Human Resource Development Group, Govt. of India, for providing fellowship towards PhD program to the first author. We are thankful to the Division of Microbiology, Indian Agricultural Research Institute, New Delhi for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, K., Prasanna, R. & Saxena, A.K. Rhizobia: a potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol 62, 425–435 (2017). https://doi.org/10.1007/s12223-017-0513-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-017-0513-z