Abstract
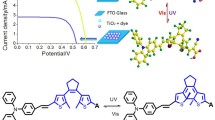
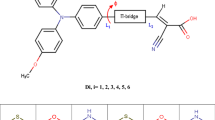
Four organic sensitizers containing quinoxaline or benzoxadiazole as an auxiliary electron acceptor in conjugated bridge were synthesized and utilized for dye-sensitized solar cells (DSSCs). It was found that the incorporation of different electron-withdrawing moieties can affect the absorption spectra, electronic properties, the interfacial interactions and then the overall conversion efficiencies significantly. Therefore, the appropriate selection of the auxiliary acceptor was important to optimize the photovoltaic performance of solar cells. Among these sensitizers, LI-44 based solar cell showed the best photovoltaic performance: a shortcircuit photocurrent density (J sc) of 13.90 mA/cm2, an open-circuit photovoltage (V oc) of 0.66 V, and a fill factor (FF) of 0.66, corresponding to an overall conversion efficiency of 6.10% under standard global AM 1.5 solar light conditions.
Similar content being viewed by others
References
O’Regan B, Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature, 1991, 353(6346): 737–740
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H. Dyesensitized solar cells. Chemical Reviews, 2010, 110(11): 6595–6663
Hardin B E, Snaith H J, McGehee M D. The renaissance of dyesensitized solar cells. Nature Photonics, 2012, 6(3): 162–169
Joly D, Pellejà L, Narbey S, Oswald F, Chiron J, Clifford J N, Palomares E, Demadrille R. A robust organic dye for dye sensitized solar cells based on iodine/iodide electrolytes combining high efficiency and outstanding stability. Scientific Reports, 2014, 4: 4033
Joly D, Pellejà L, Narbey S, Oswald F, Meyer T, Kervella Y, Maldivi P, Clifford J N, Palomares E, Demadrille R. Metal-free organic sensitizers with narrow absorption in the visible for solar cells exceeding 10% efficiency. Energy & Environmental Science, 2015, 8(7): 2010–2018
Kang X, Zhang J, O’Neil D, Rojas A J, Chen W, Szymanski P, Marder S R, El-Sayed M A. Effect of molecular structure perturbations on the performance of the D-A-p-A dye sensitized solar cells. Chemistry of Materials, 2014, 26(15): 4486–4493
Cui Y, Wu Y, Lu X, Zhang X, Zhou G, Miapeh F B, Zhu W, Wang Z S. Incorporating benzotriazole moiety to construct D-A-p-A organic sensitizers for solar cells: significant enhancement of open-circuit photovoltage with long alkyl group. Chemistry of Materials, 2011, 23(19): 4394–4401
Pei K, Wu Y, Islam A, Zhang Q, Han L, Tian H, Zhu W. Constructing high-efficiency D-A-p-A-featured solar cell sensitizers: a promising building block of 2,3-diphenylquinoxaline for antiaggregation and photostability. ACS Applied Materials & Interfaces, 2013, 5(11): 4986–4995
Lu X, Feng Q, Lan T, Zhou G, Wang Z S. Molecular engineering of quinoxaline-based organic sensitizers for highly efficient and stable dye-sensitized solar cells. Chemistry of Materials, 2012, 24(16): 3179–3187
Shi J, Chen J, Chai Z, Wang H, Tang R, Fan K, Wu M, Han H, Qin J, Peng T, Li Q, Li Z. High performance organic sensitizers based on 11,12-bis(hexyloxy) dibenzo[a,c]phenazine for dye-sensitized solar cells. Journal of Materials Chemistry, 2012, 22(36): 18830–18838
Yang J, Ganesan P, Teuscher J, Moehl T, Kim Y J, Yi C, Comte P, Pei K, Holcombe T W, Nazeeruddin M K, Hua J, Zakeeruddin S M, Tian H, Grätzel M. Influence of the donor size in D-p-A organic dyes for dye-sensitized solar cells. Journal of the American Chemical Society, 2014, 136(15): 5722–5730
Li X, Hu Y, Sanchez-Molina I, Zhou Y, Yu F, Haque S A, Wu W, Hua J, Tian H, Robertson N. Insight into quinoxaline containing D- p-A dyes for dye-sensitized solar cells with cobalt and iodine based electrolytes: the effect of p-bridge on the HOMO energy level and photovoltaic performance. Journal of Materials Chemistry A, Materials for Energy and Sustainability, 2015, 3(43): 21733–21743
Ying W, Guo F, Li J, Zhang Q, Wu W, Tian H, Hua J. Series of new D-A-p-A organic broadly absorbing sensitizers containing isoindigo unit for highly efficient dye-sensitized solar cells. ACS Applied Materials & Interfaces, 2012, 4(8): 4215–4224
Zhu W, Wu Y, Wang S, Li W, Li X, Chen J, Wang Z, Tian H. Organic D-A-p-A solar cell sensitizers with improved stability and spectral response. Advanced Functional Materials, 2011, 21(4): 756–763
Wu Y, Marszalek M, Zakeeruddin S M, Zhang Q, Tian H, Grätzel M, Zhu W. High-conversion-efficiency organic dye-sensitized solar cells: molecular engineering on D-A-p-A featured organic indoline dyes. Energy & Environmental Science, 2012, 5(8): 8261–8272
Wu Y, Zhu W. Organic sensitizers from D-p-A to D-A-p-A: effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chemical Society Reviews, 2013, 42(5): 2039–2058
Eom Y K, Choi I T, Kang S H, Lee J, Kim J, Ju M J, Kim H K. Thieno[3, 2-b] benzothiophene derivative as a new p-bridge unit in D-p-A structural organic sensitizers with over 10.47% efficiency for dye-sensitized solar cells. Advanced Energy Materials, 2015, 5(15): 1500300
Wu Y, Zhu W H, Zakeeruddin S M, Grätzel M. Insight into D-A-p- A structured sensitizers: a promising route to highly efficient and stable dye-sensitized solar cells. ACS Applied Materials & Interfaces, 2015, 7(18): 9307–9318
Chai Z, Wu M, Fang M, Wan S, Xu T, Tang R, Xie Y, Mei A, Han H, Li Q, Li Z. Similar or totally different: the adjustment of the twist conformation through minor structural modification, and dramatically improved performance for dye-sensitized solar cell. Advanced Energy Materials, 2015, 5(18): 1500846
Haid S, Marszalek M, Mishra A, Wielopolski M, Teuscher J, Moser J E, Humphry-Baker R, Zakeeruddin S M, Grätzel M, Bäuerle P. Significant improvement of dye-sensitized solar cell performance by small structural modification in p-conjugated donor-acceptor dyes. Advanced Functional Materials, 2012, 22(6): 1291–1302
Yen Y S, Chou H H, Chen Y C, Hsu C Y, Lin J T. Recent developments in molecule-based organic materials for dye-sensitized solar cells. Journal of Materials Chemistry, 2012, 22(18): 8734–8747
Liang M, Chen J. Arylamine organic dyes for dye-sensitized solar cells. Chemical Society Reviews, 2013, 42(8): 3453–3488
Koumura N, Wang Z S, Mori S, Miyashita M, Suzuki E, Hara K. Alkyl-functionalized organic dyes for efficient molecular photovoltaics. Journal of the American Chemical Society, 2006, 128(44): 14256–14257
Yamamoto T, Sugiyama K, Kushida T, Inoue T, Kanbara T. Preparation of new electron-accepting p-conjugated polyquinoxalines. Chemical and electrochemical reduction, electrically conducting properties, and use in light-emitting diodes. Journal of the American Chemical Society, 1996, 118(16): 3930–3937
Blouin N, Michaud A, Gendron D, Wakim S, Blair E, Neagu-Plesu R, Belletête M, Durocher G, Tao Y, Leclerc M. Toward a rational design of poly(2,7-carbazole) derivatives for solar cells. Journal of the American Chemical Society, 2008, 130(2): 732–742
Li H, Yang Y, Hou Y, Tang R, Duan T, Chen J, Wang H, Han H, Peng T, Chen X, Li Q, Li Z. Organic sensitizers featuring 9,10- diaryl-substituted anthracene unit. ACS Sustainable Chemistry & Engineering, 2014, 2(7): 1776–1784
Li Q, Shi J, Li H, Li S, Zhong C, Guo F, Peng M, Hua J, Qin J, Li Z. Novel pyrrole-based dyes for dye-sensitized solar cells: from rodshape to “H” type. Journal of Materials Chemistry, 2012, 22(14): 6689–6696
Li H, Hou Y, Yang Y, Tang R, Chen J, Wang H, Han H, Peng T, Li Q, Li Z. Attempt to improve the performance of pyrrole-containing dyes in dye sensitized solar cells by adjusting isolation groups. ACS Applied Materials & Interfaces, 2013, 5(23): 12469–12477
Frisch G W T M J, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas Ö, Foresman J B, Ortiz J V, Cioslowski J, Fox D J. Gaussian, Inc., Wallingford CT. 2009
Salvatori P, Marotta G, Cinti A, Anselmi C, Mosconi E, De Angelis F. Supramolecular interactions of chenodeoxycholic acid increase the efficiency of dye-sensitized solar cells based on a cobalt electrolyte. Journal of Physical Chemistry C, 2013, 117(8): 3874–3887
Tang J, Hua J, Wu W, Li J, Jin Z, Long Y, Tian H. New starburst sensitizer with carbazole antennas for efficient and stable dyesensitized solar cells. Energy & Environmental Science, 2010, 3(11): 1736–1745
Wang Q, Moser J E, Grätzel M. Electrochemical impedance spectroscopic analysis of dye-sensitized solar cells. Journal of Physical Chemistry B, 2005, 109(31): 14945–14953
Adachi M, Sakamoto M, Jiu J, Ogata Y, Isoda S. Determination of parameters of electron transport in dye-sensitized solar cells using electrochemical impedance spectroscopy. Journal of Physical Chemistry B, 2006, 110(28): 13872–13880
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qianqian Li received her B.Sc. degree from Hubei University, China in 2004, and then obtained her Ph.D. degree at Wuhan University in 2009. She is now an associate professor at Wuhan University, and her research interests are in the design and synthesis of new electric and optical functional materials.
Rights and permissions
About this article
Cite this article
Shi, J., Chai, Z., Tang, R. et al. Effect of electron-withdrawing groups in conjugated bridges: molecular engineering of organic sensitizers for dye-sensitized solar cells. Front. Optoelectron. 9, 60–70 (2016). https://doi.org/10.1007/s12200-016-0567-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12200-016-0567-6