Abstract
Two sensitizers with novel structure were designed and synthetized by introducing photochromic bisthienylethene (BTE) group into the conjugated system. Thanks to the photochromic effect the sensitizers have under ultraviolet and visible light, the conjugated bridge can be restructured and the resulting two photoisomers showed different behaviors in photovoltaic devices. This opens up a new research way for the dye-sensitized solar cells (DSSCs).
Similar content being viewed by others
Among the various processes to utilize solar energy, DSSCs that are based on highly porous nanocrystalline films of titanium dioxide (TiO2) have received considerable attention due to their high power conversion efficiency, low cost and high semiconductor stability1,2,3,4,5,6,7,8. To further improve their energy conversion efficiencies, much effort has been devoted to the optimization of components (e.g. sensitizers, electrolyte and counter electrodes) and to the design of creative novel structures of DSSCs9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24.
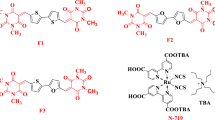
Pure organic dyes, as a major candidate of the sensitizers for DSSCs25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52, have been extensively explored with the basic D-π-A structure. The energy level of these dyes exerts a significant influence on the photovoltaic performances53. The conventional methods to adjust the orbital levels were to change their donors, π-bridges or acceptors46,47,48,49,50,51,52,53,54,55,56. It is well-known that photochromic compounds based on bisthienylethene (BTE) unit are one of the most promising materials because of their excellent fatigue resistance and thermal stability in both isomeric forms. The open- and closed-ring isomers of BTE differ from each other not only in their absorption but also in optical data storage and optical signal processing. In this work, we herein incorporated the photochromic BTE unit into D-π-A sensitizers in order to develop optical switching sensitizers for dye-sensitized solar cells. As shown in Figrue 1, the photochromic dyes based on BTE moiety (BTE-CA and BTE-CN) can form two photoisomers (coded as CNO and CNC or CAO and CAC with different acceptor) with the open or closed-ring by alternating irradiation with UV and visible light57,58,59,60. Therefore, the photoelectric conversion efficiency (PCE) of the DSSCs based on these dyes can be changed with the structure of the sensitizers tuned reversibly under irradiation of UV or visible light.
In Figure S1, CAO/CAC and CNO/CNC represent the open-ring/closed-ring forms of compounds BTE-CA and BET-CN, respectively. As shown in Figure 2a, upon alternating irradiation with UV and visible light, the sensitizers showed typical photochromic properties. When irradiated at 365 nm, the compounds showed a reduction in intensity of the absorption around 380 nm and a rise of a new absorption at 574 nm and 694 nm for BTE-CA and BTE-CN, respectively (Figure 2a and Figure S2). The low energy band appeared at 574 nm for CAC or 694 nm for CNC, arising from the charge-transfer transition, suggests the formation of large D-π-A conjugated closed-ring diarylethene, which corresponds to the colour change of the solution from colourless to bluish-purple or yellow to green (inset in Figure 2a). Comparing to CAC, the absorption spectrum of CNC extended into the near infrared region due to the stronger electron-withdrawing character of cyanoacetic acid group. Upon irradiation with visible light (λ > 500 nm), the bluish-purple or green solution bleached to colourless or yellow, indicating that the retrieving of open-ring isomer (CAO or CNO). After anchoring on TiO2 film, the λmax of CAC and CNC hypsochromically shifted to 560 nm and 589 nm, respectively, which can be ascribed to the deprotonation and aggregation of the dyes (Figure 2b).
To obtain and characterise the molecular orbital energy levels, cyclic voltammetry (CV) was employed to measure the oxidation potential of the dyes in CH2Cl2; these CV curves are shown in Figure S3. The corresponding electrochemistry data are given in Table S1 and the energy levels are demonstrated in Figure S4. The first two oxidation potentials (Eox) of different isomers, corresponding to the highest occupied molecular orbital (HOMO) and HOMO-1 levels, were converted to a normal hydrogen electrode (NHE) with ferrocene/ferrocenium (Fc/Fc+) as an external reference. The zeroth-zeroth energy (E0–0) values, defined as the optical gap of the sensitizers, were obtained from the absorption thresholds (Table S1). From above data, we found that their HOMO and LUMO levels thermodynamically matched well with the iodine/iodide redox potential value (0.4 V) and Ecb of the TiO2 electrode (0.5 V vs. NHE).
Figure 3 shows the J-V and P-V curves with the corresponding photovoltaic data summarized in Table S1. From Figure 3 and Table S1, the photocurrent density vs. voltage curves for DSSCs based on CAO, CAC, CNO and CNC were given and these cells have a solar energy to electricity conversion efficiency of 0.87% (Jsc = 2.00 mA cm−2, Voc = 602 mV, ff = 0.72), 0.30% (Jsc = 0.91 mA cm−2, Voc = 500 mV, ff = 0.65), 2.00% (Jsc = 4.42 mA cm−2, Voc = 650 mV, ff = 0.70), 0.59% (Jsc = 1.61 mA cm−2, Voc = 540 mV, ff = 0.68), respectively. In these data, the short-circuit photocurrent (Jsc) and Voc are critical parameters determining the energy conversion efficiency of the cells. While Jsc is mostly controlled by the light-harvesting and charge-injection efficiency of sensitizer, Voc is determined by the difference between the quasi-Fermi level in the TiO2 and the energy level of the redox couple in the electrolyte61. As we all know, the charge recombination between injected electrons and oxidized species in the electrolyte will result in a reduced Voc62,63,64,65,66,67,68,69,70,71.
To analyse why the sensitizers with an open-ring give better photovoltanic performances in DSSCs, the orbital distributions of different isomers (Figure S4) were achieved by density functional theory (DFT) calculations at the B3LYP/6-31G* level. As illustrated in Figure S4, the HOMO orbitals in CAO and CNO are primarily located at the π-framework of the donor part, while the electron density of the LUMOs are delocalized over the BTE unit and anchoring group. The distinct location of the HOMO and LUMO orbitals enables a good charge separation. However, for CAC and CNC, the electron density of HOMO or LUMO orbital locates at the conjunction bridges (BTE unit) and acceptors, suggesting the strong electron-donating ability of the closed-ring leads to a poor charge separation.
In summary, two new D-π-A type sensitizers for DSSCs based on BTE photochromic unit, BTE-CA and BTE-CN, were successfully synthesized and their photovoltaic performances were characterised. There are some elements for shaping their PCE performance including the variation of absorption spectroscopy, orbital distribution and CB shift following the photochromic interconversion between the different photoisomers by alternating irradiation with UV and visible light. Using their tautomeric characteristics, we first attempt to achieve a regulation of the photovoltaic performance of the sensitizer with photons of different wavelengths.
References
O'Regan, B. & Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991).
Park, S. H. et al. Stable dye-sensitized solar cells by encapsulation of N719-sensitized TiO2 electrodes using surface-induced cross-linking polymerization. Adv. Energy Mater. 2, 219–224 (2012).
Hagfeldt, A. et al. Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010).
Li, C. et al. Polyphenylene-based materials for organic photovoltaics. Chem. Rev. 110, 6817–6855 (2010).
Li, Q. et al. High-temperature solid-state dye-sensitized solar cells based on organic ionic plastic crystal electrolytes. Adv. Mater. 24, 945–950 (2012).
Li, Y. et al. Gold nanoparticles inlaid TiO2 photoanodes: a superior candidate for high-efficiency dye-sensitized solar cells. Energy Environ. Sci. 6, 2156–2165 (2013).
Chen, S. et al. A multiaddressable photochromic bisthienylethene with sequence-dependent responses: construction of an inhibit logic gate and a keypad lock. ACS Appl. Mater. Interfaces 5, 5623–5629 (2013).
Qu, S. Y., Hua, J. L. & Tian, H. New D-π-A dyes for efficient dye-sensitized solar cells. Sci. China Chem. 55, 677–697 (2012).
Kuang, D. et al. Organic dye-sensitized ionic liquid based solar cells: remarkable enhancement in performance through molecular design of indoline sensitizers. Angew. Chem. Int. ed. 47, 1923–1927 (2008).
Qin, H. et al. An organic sensitizer with a fused dithienothiophene unit for efficient and stable dye-sensitized solar cells. J. Am. Chem. Soc. 130, 9202–9203 (2008).
Yum, J. H. et al. A light-resistant organic sensitizer for solar-cell applications. Angew. Chem. Int. Ed. 48, 1576–1580 (2009).
Dentani, T. et al. Novel thiophene-conjugated indoline dyes for zinc oxide solar cells. New J. Chem. 33, 93–101 (2009).
Zhu, W. et al. Organic D-A-π-A Solar Cell Sensitizers with Improved Stability and Spectral Response. Adv. Funct. Mater. 21, 756–763 (2011).
Sun, X. et al. One-step preparation of mirror-like NiS nanosheets on ITO for the efficient counter electrode of dye-sensitized solar cells. Chem. Commun. 50, 9869–9871 (2014).
Guo, M. X. et al. A high efficiency CoCr2O4/carbon nanotubes nanocomposite electrocatalyst for dye-sensitised solar cells. Chem. Commun. 50, 7356–7358 (2014).
Pan, J. et al. A nonstoichiometric SnO2-δ nanocrystal-based counter electrode for remarkably improving the performance of dye-sensitized solar cells. Chem. Commun. 50, 7020–7023 (2014).
Weidelener, M. et al. Dithienopyrrole-based oligothiophenes for solution-processed organic solar cells. Chem. Commun. 49, 10865–10867 (2013).
Zheng, X. J. et al. Low-cost and high-performance CoMoS4 and NiMoS4 counter electrodes for dye-sensitized solar cells. Chem. Commun. 49, 9645–9647 (2013).
Yum, J. H. et al. Towards high-performance DPP-based sensitizers for DSC applications. Chem. Commun. 48, 10727–10729 (2012).
Wu, K. L. et al. Dye Molecular Structure Device Open-Circuit Voltage Correlation in Ru(II) Sensitizers with Heteroleptic Tridentate Chelates for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 134, 7488–7496 (2012).
Yella, A. et al. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 334, 629–634 (2011).
Daeneke, T. et al. Dye Regeneration Kinetics in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 134, 16925–16928 (2012).
Kuang, D. et al. Stable Mesoscopic Dye-Sensitized Solar Cells Based on Tetracyanoborate Ionic Liquid Electrolyte. J. Am. Chem. Soc. 128, 7732–7733 (2006).
Sauvage, F. et al. Effect of Sensitizer Adsorption Temperature on the Performance of Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 133, 9304–9310 (2011).
Hara, K. et al. A coumarin-derivative dye sensitized nanocrystalline TiO2 solar cell having a high solar-energy conversion efficiency up to 5.6%. Chem. Commun. 1, 569–570 (2001).
Hara, K. et al. Oligothiophene-Containing Coumarin Dyes for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. B 109, 15476–15482 (2005).
Sayama, K. et al. Photosensitization of a porous TiO2 electrode with merocyanine dyes containing a carboxyl group and a long alkyl chain. Chem. Commun. 1173–1174; 10.1039/b001517m (2000).
Hara, K. et al. Novel Conjugated Organic Dyes for Efficient Dye-Sensitized Solar Cells. Adv. Funct. Mater. 15, 246–252 (2005).
Wang, Z. S. et al. High-Light- Harvesting-Efficiency Coumarin Dye for Stable Dye-Sensitized Solar Cells. Adv. Mater. 19, 1138–1141 (2007).
Horiuchi, T., Miura, H., Sumioka, K., & Uchida, S. High Efficiency of Dye-Sensitized Solar Cells Based on Metal-Free Indoline Dyes. J. Am. Chem. Soc. 126, 12218–12219 (2004).
Ying, W. et al. New pyrido[3,4-b]pyrazine-based sensitizers for efficient and stable dye-sensitized solar cells. Chem. Sci. 5, 206–214 (2014).
Yang, J. B. et al. Influence of the Donor Size in D-π-A Organic Dyes for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 136, 5722–5730 (2014).
Velusamy, M. et al. Organic Dyes Incorporating Low-Band-Gap Chromophores for Dye-Sensitized Solar Cells. Org. Lett. 7, 1899–1902 (2005).
Kitamura, T. et al. Phenyl-Conjugated Oligoene Sensitizers for TiO2 Solar Cells. Chem. Mater. 16, 1806–1812 (2004).
Hagberg, D. P. et al. A novel organic chromophore for dye-sensitized nanostructured solar cells. Chem. Commun. 2245–2247; 10.1039/B603002E (2006).
Hagberg, D. P. et al. Tuning the HOMO and LUMO Energy Levels of Organic Chromophores for Dye Sensitized Solar Cells. J. Org. Chem. 72, 9550–9556 (2007).
Hagberg, D. P. et al. Molecular Engineering of Organic Sensitizers for Dye-Sensitized Solar Cell Applications. J. Am. Chem. Soc. 130, 6259–6266 (2008).
Tian, H. et al. Effect of Different Dye Baths and Dye-Structures on the Performance of Dye-Sensitized Solar Cells Based on Triphenylamine Dyes. J. Phys. Chem. C. 112, 1023–11033 (2008).
Kim, S. et al. Molecular Engineering of Organic Sensitizers for Solar Cell Applications. J. Am. Chem. Soc. 128, 16701–16707 (2006).
Kim, D., Lee, J. K., Kang, S. O. & Ko, J. Molecular engineering of organic dyes containing N-aryl carbazole moiety for solar cell. Tetrahedron 63, 1913–1922 (2007).
Choi, H. et al. Novel organic dyes containing bis-dimethylfluorenyl amino benzo[b]thiophene for highly efficient dye-sensitized solar. Tetrahedron 63, 3115–3121 (2007).
Choi, H. et al. Highly Efficient and Thermally Stable Organic Sensitizers for Solvent-Free Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 47, 327–330 (2008).
Tian, H. et al. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun. 3741–3743 (2007); 10.1039/b707485a.
Chen, R. et al. Effect of Tetrahydroquinoline Dyes Structure on the Performance of Organic Dye-Sensitized Solar Cells. Chem. Mater. 19, 4007–4015 (2007).
Chen, R., Yang, X., Tian, H., & Sun, L. Tetrahydroquinoline dyes with different spacers for organic dye-sensitized solar cells. J. Photochem. Photobiol. A. 189, 295–300 (2007).
Chen, B. S. et al. Donor–acceptor dyes with fluorine substituted phenylene spacer for dye-sensitized solar cells. J. Mater. Chem. 21, 1937–1945 (2011).
Gupta, A. et al. New organic sensitizers using 4-(cyanomethyl)benzoic acid as an acceptor group for dye-sensitized solar cell applications. Dyes Pigments 113, 280–288 (2015).
Xiang, W. C. et al. Cyanomethylbenzoic Acid: An Acceptor for Donor-π-Acceptor Chromophores Used in Dye-Sensitized Solar Cells. ChemSusChem 6, 256–260 (2013).
Perera, I. R. et al. Introducing manganese complexes as redox mediators for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 16, 12021–12028 (2014).
Chen, D. Y. et al. A new recognition concept using dye sensitized solar cell configuration. Chem. Commun. 47, 985–987 (2011).
Cui, Y. et al. Incorporating Benzotriazole Moiety to Construct D-A-π-A Organic Sensitizers for Solar Cells: Significant Enhancement of Open-Circuit Photovoltage with Long Alkyl Group. Chem. Mater. 23, 4394–4401 (2011).
Zhang, G. L. et al. High efficiency and stable dye-sensitized solar cells with an organic chromophore featuring a binary π-conjugated spacer. Chem. Commun. 2198–2200; 10.1039/B822325D (2009).
Wu, W. J. et al. Efficient and stable dye-sensitized solar cells based on phenothiazine sensitizers with thiophene units. J. Mater. Chem. 20, 1772–1779 (2010).
Mao, J. Y. et al. Stable Dyes Containing Double Acceptors without COOH as Anchors for Highly Efficient Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 51, 9873–9876 (2012).
Zhu, W. H. et al. Unprecedented Stability of a Photochromic Bisthienylethene Based on Benzobisthiadiazole as an Ethene Bridge. Angew. Chem. Int. Ed. 50, 10986–10990 (2011).
Wu, Y. Z. et al. Hexylthiophene-Featured D-A-π-A Structural Indoline Chromophores for Coadsorbent-Free and Panchromatic Dye-Sensitized Solar Cells. Adv. Energy Mater. 2, 149–156 (2012).
Beydoun, K., Boixel, J., Guerchais, V. & Doucet, H. Direct arylation of dithienylperfluorocy- clopentenes via palladium-catalysed C-H bond activation: a simpler access to photoswitches. Catal. Sci. Technol. 2, 1242–1248 (2012).
Li, Z. Y. et al. Synthesis and Properties of Photochromic Diarylethene Containing N-Salicylideneaniline Units. Mol. Cryst. Liq. Cryst. 557, 84–89 (2012).
Belfield, K. D. et al. Photophysical Properties and Ultrafast Excited-State Dynamics of a New Two-Photon Absorbing Thiopyranyl Probe. J. Phys. Chem. C 117, 11941–11952 (2013).
Luchita, G. et al. Efficient Photochromic Transformation of a New Fluorenyl Diarylethene: One- and Two-Photon Absorption Spectroscopy. ACS Appl. Mater. Interfaces 3, 3559–3567 (2011).
Frank, A. J., Kopidakis, N. & Lagemaat, V. D. Electrons in nanostructured TiO2 solar cells: Transport, recombination and photovoltaic properties. J. Coord. Chem. Rev. 248, 1165–1179 (2004).
Kay, A. & Grätzel, M. Artificial photosynthesis. 1. Photosensitization of titania solar cells with chlorophyll derivatives and related natural porphyrins. J. Phys. Chem. 97, 6272–6277 (1993).
Huang, S. Y. et al. Charge Recombination in Dye-Sensitized Nanocrystalline TiO2 Solar Cells. J. Phys. Chem. B 101, 2576–2582 (1997).
Kusama, H. & Arakawa, H. Influence of aminothiazole additives in I−/I3− redox electrolyte solution on Ru(II)-dye-sensitized nanocrystalline TiO2 solar cell performance. Sol. Energy Mater. Sol. Cells 82, 457–465 (2004).
Zaban, A. & Ferrere, S. Relative Energetics at the Semiconductor/Sensitizing Dye/Electrolyte Interface. J. Phys. Chem. B 102, 452–460 (1998).
Kang, T. S. et al. Enhanced Stability of Photocurrent-Voltage Curves in Ru(II)-Dye-Sensitized Nanocrystalline TiO2 Electrodes with Carboxylic Acids. J. Electrochem. Soc. 147, 3049–3053 (2000).
Nazeeruddin, M. K. et al. Conversion of light to electricity by cis-X2bis(2,2'-bipyridyl-4,4'- dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN− and SCN−) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 115, 6382–6390 (1993).
Nakade, S. et al.Role of Electrolytes on Charge Recombination in Dye-Sensitized TiO2 Solar Cell (1): The Case of Solar Cells Using the I−/I3− Redox Couple. J. Phys. Chem. B 109, 3480–3487 (2005).
Schlichthörl, G., Huang, S. Y., Sprague, J., & Frank, A. J. Band edge movement and recombination kinetics in dye-sensitized nanocrystalline TiO2 solar cells: a study by intensity modulated photovoltage spectroscopy. J. Phys. Chem. B 101, 8141–8155 (1997).
Boschloo, G. et al. Optimization of dye-sensitized solar cells prepared by compression method. J. Photochem. Photobiol. A 148, 11–15 (2002).
Kusama, H., Konishi, Y., Sugihara, H., & Arakawa, H. Influence of alkylpyridine additives in electrolyte solution on the performance of dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells 80, 167–179 (2003).
Acknowledgements
W.-J. Wu appreciates Prof. H. Tian very much for his helpful discussion and valuable comments. This work was supported by NSFC/China (2116110444, 21172073, 91233207 and 21372082) and the National Basic Research 973 Program (2013CB733700), the Fundamental Research Funds for the Central Universities (WJ1315025), Scientific Committee of Shanghai (14ZR1409700) and the Science Fund for Creative Research Groups (21421004).
Author information
Authors and Affiliations
Contributions
W.J.W. wrote the main manuscript and Figure 1–3. J.X.W. and J.Y.J. synthesized the compounds. Z.W.Z. prepared the photovoltaic devices and characterized the photoelectric properties. Y.H. and Q.Z. provided the theoretical calculation and guidance. J.L.H. wrote parts of discussion section. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wu, W., Wang, J., Zheng, Z. et al. A strategy to design novel structure photochromic sensitizers for dye-sensitized solar cells. Sci Rep 5, 8592 (2015). https://doi.org/10.1038/srep08592
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08592
- Springer Nature Limited