Abstract
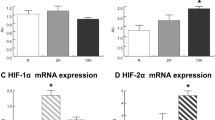
While numerous maladies are associated with hypobaric hypoxia, muscle protein loss is an important under studied topic. Hence, the present study was designed to investigate the mechanism of muscle protein loss at HH. SD rats were divided into normoxic rats, while remaining rats were exposed to simulated hypoxia equivalent to 282-torr pressure (equal to an altitude of 7620 m, 8% oxygen), at 25 °C for 6, 12, and 24 h. Post-exposure rats were sacrificed and analysis was performed. Ergo, muscle loss-related changes were observed at 12 and 24 h post-HH exposure. An increased reactive oxygen species production and decreased thiol content was observed in HH-exposed rats. This disturbance caused substantial protein oxidative modification in the form of protein carbonyl content and advanced oxidation protein products. The analysis showed increase levels of bityrosine, oxidized tryptophan, lysine conjugate, lysine conjugate with MDA, protein hydroperoxide, and protein-MDA product. These changes were also in agreement with increase in lipid hydroperoxides and MDA content. HSP-70 and HSP-60 were upregulated significantly, and this finding is corroborated with increase in ER stress biomarker, GRP-78. Overloading of cells with misfolded proteins further activated degradative machinery. Consequently, pro-apoptotic signaling cascade, caspase-3, and C/EBP homologous protein were also activated in 24-h HH exposure. Release of tryptophan and tyrosine was also increased with 24-h HH exposure, indicated protein degradation. Elevation in resting intracellular calcium ion, [Ca2+]i, was also observed at 12- and 24-h HH exposure. The present study provides a detailed mechanistic representation of muscle protein loss during HH exposure.








Similar content being viewed by others
Abbreviations
- HH:
-
Hypobaric hypoxia
- GRP:
-
Glucose regulating protein
- HSP:
-
Heat shock protein
- AOPP:
-
Advanced oxidation protein product
- MDA:
-
Malondialdehyde
References
Alderton JM, Steinhardt RA (2000) Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 275:9452–9460
Baehr LM, West DWD, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC (2016) Age related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Ageing 8:27–146
Bailey DM, Davies B, Young IS (2001) Intermittent hypoxic training: implications for lipid peroxidation induced by acute normoxic exercise inactive men. Clin Sci (Lond) 101:465–475
Barreir E, Hussain SNA (2010) Protein carbonylation in skeletal muscles: impact on function. Antioxid Redox Signal 12:417–429
Bartoli M, Richard I (2005) Calpains in muscle wasting. Int J Biochem Cell Biol 37:2115–2133
Bharadwaj H, Prasad J, Pramanik SN, Kishnani S, Zachariah T, Chaudhary KL, Sridharan K, Srivastava KK (2000) Effect of prolonged exposure to high altitude on skeletal muscles of Indian soldiers. Def Sci J 50:167–176
Bligh J, Johnson KG (1973) Glossary of terms for thermal physiology. J Appl Physiol 35:941–961
Bondy SC (1996) Evaluation of free radical-initiated oxidant events within the nervous system. In: Perez-Polo JR (ed) Methods in neuroscience 30. Academic Press, San Diego, pp 243–259
Botta A, Malena A, Loro E, Del Moro G, Suman M, Pantic B, Szabadkai G, Vergani L (2013) Altered Ca2+ homeostasis and endoplasmic reticulum stress in myotonic dystrophy type 1 muscle cells. Genes (Basel) 4:275–292
Bradford MM (1994) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Butterfield GE (1990) The adaptations. In: Sutton JR, Coates G, Remmers JE (eds) Hypoxia. Elements of energy balance at altitude. Decker BC, Inc, Philadelphia, pp 88–93
Butterfield DA, Stadtman ER (1997) Protein oxidation processes in aging brain. Adv Cell Aging Gerontol 2:161–191
Calbet JA, Robach P, Lundby C (2009) The exercising heart at altitude. Cell Mol Life Sci 66:3601–3613
Castegna A, Drake J, Pocernich C, Butterfield DA (2003) Protein carbonyl levels—an assessment of protein oxidation. In: Hensley K, Floyd RA (eds) Methods in pharmacology and toxicology: Methods in biological oxidative stress. © Humana Press Inc., Totowa
Cathcart R, Schwiers E, Ames BN (1983) Detection of pico mole levels of hyderoperoxides using fluorescent dichlorofluoroscein assay. Anal Biochem 134:111–116
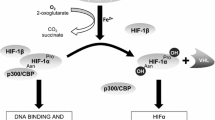
Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia. A mechanic of O2 sensing. J Biol Chem 275:25130–25138
Chaudhary P, Suryakumar G, Prasad R, Singh SN, Ali S, Ilavazhagan G (2012a) Effect of acute hypobaric hypoxia on skeletal muscles protein turnover. Al Ameen J Med Sci 5:355–361
Chaudhary P, Suryakumar G, Prasad R, Singh SN, Ali S, Ilavazhagan G (2012b) Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin–proteasome pathway and calpains. Mol Cell Biochem 364:101–113
D’Hulst G, Jamart C, Van Thienen R, Hespel P, Francaux M, Deldicque L (2013) Effect of acute environmental hypoxia on protein metabolism in human skeletal muscle. Acta Physiol 208:251–264
Dousset N, Ferretti G, Taus M, Valdiguie P, Curatola G (1994) Fluorescence analysis of lipoprotein peroxidation. Methods Enzymol 233:459–469
Eaton P (2006) Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med 40:1889–1899
Edwards LM, Murray AJ, Tyler DJ, Kemp GJ, Holloway, Robbins PA, Neubauer S, Levett D, Montgomery HE, Grocott MP, Clarke K (2010) The effect of high-altitude on human skeletal muscle energetics: P-MRS results from the Caudwell Xtreme Everest expedition. PLoS One 5:5
Ferreira LF, Reid MB (2008) Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol 104:853–860
Giulivi C, Davies KJ (1993) Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19S) proteasome. J Biol Chem 268:8752–8759
Giulivi C, Davies KJA (1994) Dityrosine: a marker for oxidatively modified proteins and selective proteolysis. Methods Enzymol 233:363–371
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The Calpain system. Physiol Rev 83:731–801
Grintzalis K, Zisimopoulos D, Grune T, Weber D, Georgiou CD (2013) Method for the simultaneous determination of free/protein malondialdehyde and lipid/protein hydroperoxides. Free Radic Biol Med 59:27–35
Gusow K, Szabelski M, Rzeska A, Karolczak J, Sulowska H, Wiczk W (2002) Photophysical properties of tyrosine at low pH range. Chem Phys Lett 362:519–526
Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT (2005) Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell 1(6):401–408
Heerlein K, Schulze A, Hotz L, Bartsch P, Mairbaurl H (2005) Hypoxia decreases cellular ATP demand and inhibits mitochondrial respiration of a549 cells. Am J Respir Cell Mol Biol 32:44–51
Hepple RT, Qin M, Nakamoto H, Goto S (2008) Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am J Physiol Regul Integr Comp Physiol 295:R1231–R1237
Hochachka PW, Buck LT, Doll CJ, Land SC (1996) Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci U S A 93:9493–9498
Hoppler H, Desplanches D (1992) Muscle structural modifications in hypoxia. Int J Sport Med 1:S166–S168
Hoppler H, Vogt M (2001) Muscle tissue adaptations to hypoxia. J Exp Biol 204:3133–3139
Hoppler H, Kleinert E, Claassen C, Howald H, Kayer SR, Cerretelli P (1990) Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sport Med 11:S3–S9
Jain K, Suryakumar G, Prasad R, Ganju L (2013) Differential activation of myocardial ER stress response: a possible role in hypoxic tolerance. Int J Cardiol 168:4667–4677
Kikuchi D, Tanimoto K, Nakayama K (2016) CREB is activated by ER stress and modulates the unfolded protein response by regulating the expression of IRE1a and PER. Biochem Biophys Res Commun 469:243–250
Koritzinsky M, Magagnin MG, Van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW et al (2006) Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J 25:1114–1125
Kraggerud SM, Sandvik JA, Pettersen EO (1995) Regulation of protein synthesis in human cells exposed to extreme hypoxia. Anticancer Res 15:683–686
Kramerova I, Kudryashova E, Venkatraman G, Spencer MJ (2005) Calpain 3 participates in sarcomere remodeling by acting upstream of the ubiquitin-proteasome pathway. Hum Mol Genet 14:2125–2134
Lemus L, Goder V (2014) Regulation of endoplasmic reticulum-associated protein degradation (ERAD) by ubiquitin. Cell 3(3):824–847
Levett DZ, Radford EJ, Menassa DA, Graber EF, Morash AJ, Hoppeler H, Clarke K, Martin DS, Ferguson-Smith AC, Montgomery HE, Grocott MP, Murray AJ, Caudwell Xtreme Everest Research Group (2012) Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. FASEB J 26(4):1431–1441. doi:10.1096/fj.11-197772
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified protein. Methods Enzymol 186:464–478
Li SY, Gomelsky M, Duan J et al (2004) Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. J Biol Chem 279:11244–11252
Magalhaes J, Ascensao A, Soares JM, Neuparth MJ, Ferreira R, Oliveira J, Amado F, Duarte JA (2004) Acute and severe hypobaric hypoxia-induced muscle oxidative stress in mice: the role of glutathione against oxidative damage. Eur J Appl Physiol 91:185–191
Magalhaes J, Ascensao A, Soares JMC, Ferreira R, Neuparth MJ, Marques F, Duarte JA (2005) Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J Appl Physiol 99:1247–1253
Martinelli M, Winterhalder R, Cerretelli P, Howald H, Hoppeler H (1990) Muscle lipofuscin content and satellite cell volume is increased after high altitude exposure in humans. Experientia 46:672–676
Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M, Costelli P (2008) Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med 44:584–593
Mathieu-Costello O (2001) Muscle adaptation to altitude: tissue capillarity and capacity for aerobic metabolism. High Alt Med Biol 2:413–425
Meder W, Fink K, Gothert M (1997) Involvement of different calcium channels in K+- and veratridine-induced increases of cytosolic calcium concentration in rat cerebral cortical synaptosomes. Naunyn Schmiedeberg’s Arch Pharmacol 356:797–805
Menconi M, Fareed M, O’Neal P, Poylin V, Wei W, Hasselgren PO (2007) Role of glucocorticoids in the molecular regulation of muscle wasting. Crit Care Med 35:S602–S608
Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM, Lamb GD (2013) Ca2+-dependent proteolysis of junctophilin-1 and junctophilin-2 in skeletal and cardiac muscle. J Physiol 591:719–729
Rathor R, Sharma P, Suryakumar G, Ganju L (2015) A pharmacological investigation of Hippophae salicifolia (H S) and Hippophae rhamnoides Turkestanica (H R T) against multiple stress (C-H-R): an experimental study using rat model. Cell Stress and Chaperones 20:821–831
Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi AAA, Raden D, Kaufman RJ (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4:e374
Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta 1833:3460–3470
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound and non-protein sulfhydrylgroups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Shimoda LA, Undem C (2010) Interactions between calcium and reactive oxygen species in pulmonary arterial smooth muscle responses to hypoxia. Respir Physiol Neurobiol 174:221–229
Singh SN, Vats P, Kumria MM, Ranganathan S, Shyam R, Arora MP, Jain CL, Sridharan K (2001) Effect of high altitude (7,620 m) exposure on glutathione and related metabolism in rats. Eur J Appl Physiol 84:233–237
Smith IJ, Dodd SL (2007) Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signalling pathway in rat diaphragm muscle. Exp Physiol 92:561–573
Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C (2013) When ER stress reaches a dead end. Biochim Biophys Acta 1833:3507–3517
Van der Vlies D, Makkinje M, Jansens A, Braakman I, Verkleij AJ, Wirtz KW, Post JA (2003) Oxidation of ER resident proteins upon oxidative stress: effects of altering cellular redox/antioxidant status and implications for protein maturation. Antioxid Redox Signal 15:381–387
Vigano A, Ripamonti M, De Palma S, Capitanio D, Vasso M, Wait R, Lundby C, Cerretelli P, Gelfie C (2008) Protein modulation in human skeletal muscles in the early phase of adaptation to hypobaric hypoxia. Proteomics 8:4668–4679
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313
Xie P, Duan Y, Guo X, Hu L, Yu M (2015) Sal a attenuates hypoxia-induced endothelial endoplasmic reticulum stress and apoptosis via down-regulation of VLDL receptor expression. Cell Physiol Biochem 35:17–28
Yoshida H, Haze K, Yanagi H, Yura T, Mori K (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose regulated proteins: involvement of basic leucine zipper transcription factors. J Biol Chem 273:3741–3749
Acknowledgements
The authors are thankful to Dr. (Mrs.) Shashi Bala Singh, Director, DIPAS, for her constant support and encouragement. One of the authors, Ms. Akanksha Agrawal, is thankful for obtaining Senior Research Fellowship from DRDO.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 85 kb)
Rights and permissions
About this article
Cite this article
Agrawal, A., Rathor, R. & Suryakumar, G. Oxidative protein modification alters proteostasis under acute hypobaric hypoxia in skeletal muscles: a comprehensive in vivo study. Cell Stress and Chaperones 22, 429–443 (2017). https://doi.org/10.1007/s12192-017-0795-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-017-0795-8