Abstract
Inorganic scale deposits are a major water-related problem encountered in producing oil and gas wells. The harshness of scale deposits is dependent on the field operating conditions. Scale deposits can vary from mild scaling tendencies to extreme. In general, the scale deposit will cause a reduction in formation pores, declining productivity and eventually blockage of the wellbore and hence unexpected downtime if it is allowed to persevere. To overcome this, the productivity of an oil and gas well is ensured by handling scale deposits via removal or prevention methods. Scale prevention is the best and cost-effective method for handling scale deposits that ensures production continuity. Inhibition through “threshold” scale inhibitor treatment is the most common method that is proven to prevent or reduce likely deposits. This paper examines the art of synthetic scale inhibitors, in particular, threshold scale inhibitors in oil and gas production. It discusses the chemistry of those inhibitors, inhibition mechanisms, treatment methods and key properties for their applications. It also highlights the chemistry of the synthetic routes often used to produce them in the laboratory and/or industry. Finally, it highlights the environmental concerns for the applicability of threshold scale inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Salts which are soluble in natural waters, for instance, sea waters and formation waters, tend to deposit as scales when the concentrations of such salts exceed their saturation points during processing, treatment or use of the water (Crabtree et al. 1999; Hart and Rudie 2006; Jamero et al. 2018; Jonathan 2009). Scale formation is typical in aqueous systems where the systems are saturated with scaling ions or the concentrations of the scaling ions are raised to exceed the solubility for a particular salt at operating conditions. Typical aqueous systems where scale formation is commonly encountered and poses problems during operations are boiler water, slag cooling water, industrial cooling water used as a single pass or recycled, and oil well water to mention few (Jamero et al. 2018; Liu et al. 2012b; Olajire 2015). Concentrations of scaling ions, temperature, pressure, pH and salinity are the main factors influencing mineral scale formation in the petroleum industry (Amiri and Moghadasi 2013; Chauhan et al. 2015; Dyer and Graham 2002; Hasson and Semiat 2006; Kodel et al. 2012; Wildebrand et al. 2007). Changes in these factors will alter the scale solubility which in turn affects the kinetics and thermodynamics of the scale formation. The scaling potential influenced by these factors can be simply or complexly explained. More detailed information is described elsewhere (Al-Roomi and Hussain 2016; Amiri and Moghadasi 2013; Dyer and Graham 2002; Moghadasi et al. 2008). Other driving forces for scale formation are corrosion and dissolved gases such as H2S and CO2 (Kamal et al. 2018); these affect the formation of sulfides and other scales like carbonates and hydroxides. Also, the presence of nucleating sites in the brine system and/or on the surfaces of the equipment where scale crystals have already formed influences the scale formation. Moreover, if the brine system is treated with a scale inhibitor, the effectiveness of the scale inhibitor also determines the rate and the amount of scale formation (Hasson et al. 1998).
In general, the scale formation process has been reported to involve four phases (Al-Roomi and Hussain 2016): aggregation, nucleation, crystal growth, and agglomeration. Aggregation occurs after the brine system is supersaturated with scaling ions, cationic and anionic species collide to form ion pairs in solution and subsequently form micro-aggregates. The micro-aggregates develop into nucleation centers for crystallization which lead to the formation of micro-crystals in the nucleation stage. These micro-crystals develop into larger micro-crystals and eventually fuse to form adherent macro-crystals as they agglomerate and/or absorb to surfaces in crystal growth stage. The agglomeration stage is marked with the growing of macro-crystals into a scale film on a surface and eventually into a scale deposit via the adsorption of additional scaling ions from solution.
In oil and gas aqueous systems, \({\text{Ca}}^{2 + }\), \({\text{Ba}}^{2 + }\), and \({\text{Sr}}^{2 + }\) are scaling cations often encountered (García et al. 2005; Jonathan 2009; Kodel et al. 2012; Sorbie and Mackay 2000); \({\text{Ca}}^{2 + }\) ions deposit as calcium carbonate and/or calcium sulfate, whereas \({\text{Ba}}^{2 + }\) and \({\text{Sr}}^{2 + }\) ions commonly deposit as barium sulfate and strontium sulfate, respectively. Sulfate scales are often attributed to mixing of incompatible waters such as seawater where the sulfate ion concentration is high and formation water where the concentration(s) of \({\text{Ca}}^{2 + }\), \({\text{Ba}}^{2 + }\), and/or \({\text{Sr}}^{2 + }\) ions are/is high (Kodel et al. 2012; Sorbie and Mackay 2000). For instance, \({\text{BaSO}}_{4}\) is formed when the seawater sulfate ion concentration is high and is injected into offshore reservoirs for pressure maintenance where formation water has a high barium ion concentration. Carbonate scale (\({\text{CaCO}}_{3}\)) is generally attributed to the loss of carbon dioxide gas (CO2) from the water to the hydrocarbon phase(s) as pressure falls; the process is referred as a self-scaling (Dyer and Graham 2002). The less common scales include sulfides and hydroxides of iron which are generally due to corrosion (Jonathan 2009; Tomson et al. 2003).
The adverse effects posed by scale formation during operations depend on the type of scale and the nature of the system in which the scale is formed. In petroleum production systems, scale deposits generally can coat well systems and equipment such as rock formation, perforations, production tubing, pumps, casing, valves, and downhole completion equipment (García et al. 2005; Jonathan 2009). In so doing, thereby declined production due to restricted flow assurance (Zhang et al. 2015) caused by the reduction in pores, equipment downtime, and equipment damage. Also, scale deposits cause additional costs due to the use of expensive cleaning chemicals and labor costs associated with cleaning practices. Moreover, scale deposits reduce heat transfer as an insulating layer of mineral scale is formed and coated on the surfaces. Because of these problems, scale formation should be handled wisely; prevention is better than cure!
Several conventional techniques have been applied to stop or minimize the likely scale formation in oilfields (Graham et al. 1998; Olajire 2015; Sorbie et al. 1994; Vetter 1973). All of these have merits and disadvantages during operations. Application of scale inhibitors is proven to be an economic and cost-effective approach for combatting scale deposition in many fields. Until now, many chemicals [naturally occurring (Chaussemier et al. 2015), semisynthetic (Wang et al. 2017) or synthetic (Issabayev et al. 2018; Liu et al. 2012a)] have been proposed and successfully tested in the laboratories and/or in the field as scale inhibitors, and many of them have even been patented (Amjad and Demadis 2015). Widely available commercial scale inhibitors at present include aminotrimethylene phosphonic acid (ATMP), ethylenediaminetetramethylene phosphonic acid (EDTMP), diethylenetriaminepentamethylene phosphonic acid (DETPMP), bis(hexamethylene) triaminepenta(methylene phosphonic acid) (BHMT), pentaethylenehexamineoctakismethylene phosphonic acid (PEHOMP), 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP), and phosphino-carboxylic acids (PCA) (Laing et al. 2003; Martinod et al. 2008; Sorbie and Laing 2004; Yang et al. 2017). Others commercial scale inhibitors include polycarboxylate compounds such as homopolymaleic acid, poly(maleic-co-acrylic) acid, polyaspartic acid, and polyepoxysuccinic acid (Amjad and Koutsoukos 2014; Huang et al. 2018; Roweton et al. 1997; Wang et al. 2018; von Bonin 1983; Fukumoto and Moriyama 1987), to list few.
These inhibitors are customarily introduced into production fields where scaling is likely via continuous injection (Graham et al. 1998) or squeeze techniques. Continuous injection is preferred and considered to be most effective in particular for scale inhibitors that adsorb poorly onto the formation and where the reservoir contains non-carbonate substrates (Jonathan 2009). Nevertheless, its application is discouraged by lower availability and the expense of the facilities. Downhole squeeze (adsorption or precipitation) treatments are mostly preferred in the field and are widely practiced (Amjad and Demadis 2015; Crabtree et al. 1999). Squeeze treatments provide long-lasting inhibition particularly in carbonate reservoirs and are considered as cost-effective treatments. However, the treatments are ineffective for non-carbonate reservoirs and may cause formation damage (Graham et al. 1998; Moghadasi et al. 2004). Moreover, squeeze treatments are non-economic for scale inhibitors that adsorb poorly to the formation substrate or that whose salts are easily leached (Rabaioli and Lockhart 1996).
Scale inhibitors may prevent or retard the scaling process via sequestration of scaling cations or through intermediate scale formation phases via threshold inhibition, nucleation inhibition, crystal distortion, and/or dispersion mechanisms (Abdel-Aal and Sawada 2003; Crabtree et al. 1999; Issabayev et al. 2018; Sorbie and Laing 2004). Sequestrants such as EDTA and citric acid retard the scaling process through chelating/sequestrating the scaling cations (Crabtree et al. 1999). This is costly because the reactions are “stoichiometric,” (for example, one mole of EDTA chelates two moles of scaling calcium ions), thereby requiring large amounts of chelants for effective prevention. A much more cost-effective chemical treatment is to use a “threshold” scale inhibitor (Liu et al. 2012b), that is, one which inhibits at a concentration well below equimolar amounts. Threshold scale inhibitors effectively inhibit mineral scale growth at concentrations of 1000 times less than a balanced stoichiometric ratio of scaling cations. This paper reviews the state of the art of synthetic scale inhibitors; it discusses their applicability in the petroleum industry, the art of oilfield scale treatments, inhibition mechanisms, synthetic routes, and environmental concerns on their applications.
2 Scale inhibitors
Relatively low concentrations of water-soluble inorganic and organic compounds are known to reduce the rate of scale formation in producing wells in the petroleum industry. A chemical compound that prevents or retards mineral scale from forming in brines saturated with a pair of scaling ions (i.e., cations and anions) by chelating scaling cations and/or modifying the growing scale crystal is referred as a “scale inhibitor.” A scale inhibitor can simply be defined as a chemical substance which, when added in small amounts into the brine system, reduces or prevents scale from forming. Scale inhibitors generally contain many similar or different active functional groups that are capable of bonding (strongly or weakly) with the scaling cations or the forming nuclei or the growing crystal (Sorbie and Laing 2004; Tomson et al. 2003), thereby holding them in aqueous solutions. Scale inhibitors are used in oilfields to control or prevent scale formation in the production conduit or completion system. At present, a wide range of naturally occurring or synthetic or semisynthetic chemicals are known that can be applied as scale inhibitors. They include inorganic phosphates, organophosphorus compounds, and organic polymers (Olajire 2015).
2.1 Scale inhibitor treatment methods
The treatment of oil wells with scale inhibitors has been undertaken by a number of techniques. Continuous injection, batch treatment, and squeeze treatment (Graham et al. 1998; Sorbie et al. 1994; Sorbie and Gdanski 2005; Vazquez et al. 2016a) are three main methods which have been applied in oil well treatments. Squeeze treatment is the predominant method in the oil and gas industry. The merits and demerits of each method are summarized in Table 1.
2.1.1 Continuous injection
Continuous injection of chemical inhibitors into injection wells is one of the methods that have been practiced to prevent scale formation in petroleum producing wells (Graham et al. 1998). In this method, an aqueous solution of scale inhibitors is continuously injected through a downhole injection point in the completion. The scale inhibitor is added at the point of turbulence to achieve uniform mixing. During the addition, a constant supply of the scale inhibitor is maintained at a controlled rate. The specific inhibitor concentration needed to inhibit scale formation is closely monitored giving a very efficient use of the chemical inhibitor.
In fact, the inhibitor will tend to adsorb on the rock matrix until the absorptive capacity of the rock is reached. The inhibitor concentration in the solution is lessened before attainment of adsorptive capacity. The time taken for the rock matrix to attain its adsorptive capacity is called its retardation time, at this time the inhibition is ineffective. The retardation time will depend on injected inhibitor concentration, inter-well spacing/distances, rocky matrix type, the brine chemistry, etc. (Mackay 2005). The retardation time may increase with an increase in inter-well spacing, rock inhibitor retention, and with a decrease in inhibitor concentration; an informative discussion is found elsewhere (Mackay 2005). Continuous downhole chemical injection is preferred for scale inhibitors that poorly adsorb to the formation (Jonathan 2009). The method is considered to be more effective; however, its application is discouraged by lower availability and the expense of the necessary facilities.
2.1.2 Batch treatment
In this case, a large quantity of scale inhibitor is added periodically and used for an extended period of time. Large amounts of inhibitors are pumped into the tubing at the top, and then the inhibitors are displaced to the bottom of the tubing with the fluids in the oil well. During batch treatment, the well is closed for a specific time before production resumes.
2.1.3 Squeeze treatment
The method most often used for delivering inhibiting solutions to the scaling brine is the “squeeze treatment” (Olajire 2015; Sorbie and Gdanski 2005; Vazquez et al. 2016a, b), which is normally the best option in the case of reservoir treatments intended to protect the wellbore. The term “squeeze treatment” usually means any treatment of an unfractured producing well performed by injecting a chemical inhibitor (i.e., corrosion or scale inhibitor) into the wellbore of the producing well to place the inhibitor into the formation. In a “squeeze treatment,” a scale inhibitor solution whose concentration is normally between 2.5% and 20% by weight (Jordan et al. 1995; Kumar et al. 2018) is forced (hence the “squeeze” name) into the formation through a producer well hole after a pre-flush. The squeezing process usually includes over-flushing the treated zone with water. This moves the chemicals deeper into the formation allowing further retention on clean rocks. The over-flush stage is followed by a shut-in stage which is a soak period that enables the chemicals to be retained at a higher concentration. After over-flush and shut-in, the oil well production is resumed and the entrainment of the inhibitors in the produced water protects the producing wellbore and downhole equipment from mineral scale formation (Vazquez et al. 2016a).
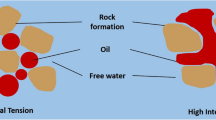
Squeezed scale inhibitor is anticipated to be slowly leached or washed back to the surface of the formation by the production water at the required minimum concentration to prevent scale formation both in the well and in the vicinity of the wellbore, as shown in Fig. 1. The scale inhibitor should leach in such a way that its concentration in the produced water phase is at or above the minimum inhibition concentration (MIC) needed to prevent scaling; more specifically 1–50 ppm (Sorbie et al. 1994; Vazquez et al. 2016a), as shown in Fig. 2. The scale inhibitor will adsorb on the formation rock, thereby avoiding the washing out of the added chemical before it has acted as desired. It is preferable if the chemical inhibitor is positioned far enough into the formation so that its desorption or other releases will be gradual, thus keeping the pores and passages for the oil open and/or the equipment and piping free of corrosion. Depending on the inhibitor retention and release properties in the formation, the effect of this treatment may last from 1 month to about 24 months. For economic reasons, a prolonged period of protection from scale deposition is obviously preferable.
Schematic representation of the field scale inhibitor squeeze treatment. a The process of injecting chemical inhibitor into the formation. b The process of returning inhibitor after the shut-in period (after Zhang et al. 2016b)
2.1.3.1 Adsorption and precipitation (phase separation) squeezes
Chemical inhibitor squeeze treatments can be of two types: either adsorption squeeze or precipitation squeeze (Andrei and Malandrino 2003; Jordan et al. 1995; Kan et al. 2005; Khormali et al. 2017; Sutherland and Jordan 2016). In the case of adsorption squeeze, the chemical inhibitor is intended to adsorb onto the rock by a physicochemical process. Adsorption of inhibitors is anticipated to occur through electrostatic and van der Waals interactions between the inhibitor and formation minerals. The interaction may be described by adsorption isotherms, which are associated with the interaction between the scale inhibitor and reservoir rock matrices. The adsorption isotherms determine an existing equilibrium between the amount of scale inhibitor adsorbed onto the rock substrate and the scale inhibitor concentration in the bulk aqueous phase. The adsorption isotherms are a function of several factors such as the pH, temperature, rock substrate, brine strength and composition, molecular weight and the inhibitor type as well (Jordan et al. 1995; Sorbie and Gdanski 2005). The adsorption isotherms for squeeze treatment are commonly explained in terms of Langmuir and Freundlich isotherms (Shuler 1993; Sorbie and Gdanski 2005). The retention of an inhibitor in the formation by the adsorption process is said to be most effective in sandstone formations (Vazquez et al. 2016a). Treatment lifetimes can be from 3 to 6 months.
On the other hand, the chemical inhibitor precipitates (or phase separates) within the pores in the formation onto the rock surfaces for a precipitation squeeze (Jordan et al. 1995; Sorbie and Gdanski 2005; Vazquez et al. 2016a). In this technique, the scale inhibitor reacts or is reacted to form an insoluble salt complex which precipitates in the pores in the formation rock. For instance, phosphonate scale inhibitors can be precipitated as calcium salts. Inhibitors such as phosphate esters are not suitable because of forming soluble calcium salts or salt complexes. During squeezing, an acidic solution of a phosphonate is squeezed down a wellbore into a limestone or dolomite-containing reservoir. The acid reacts with the carbonate from the limestone or dolomite. In so doing, the acid is neutralized and calcium ions are released into the solution, thereby reacting with the inhibitor to form the insoluble salt. The reaction between the scale inhibitor and calcium ions is illustrated in Fig. 3.
In the reaction above, the inhibitor is precipitated with polyvalent ions, generally calcium ions. On adjusting the pH and increasing a calcium ion concentration, precipitation of scale inhibitor takes place within the pore spaces of the rock. Following precipitation, the well is returned to production. The precipitation with other ions, such as iron or chromium, has been proposed. Calcium salts of phosphonic acid, phosphino-polycarboxylic acid, polymaleic acid, polyacrylic acid, and/or copolymeric acid scale inhibitor are also useful. The precipitation inhibitor is intended to place more inhibitor per squeeze, and to extend the treatment lifetime (Jordan et al. 1995; Sorbie and Gdanski 2005); treatment lifetimes generally exceed 1 year. Thus, precipitation squeezes are generally superior to adsorption squeezes. The common drawback of precipitation squeezes is that the inhibitor tends to have a limited transport distance inside the formation, which results in a reduced surface area of the formation to be treated (Zhang et al. 2016b).
As precipitation squeezes are usually superior to adsorption squeezes, thus, a chemical inhibitor whose calcium salt has a very low solubility should be a superior squeeze chemical. The solubility of the inhibitor salt should desorb so that the produced inhibitor concentration in the produced water phase is just above the MIC. In spite of the many challenges with squeeze treatments, it is one of the most effective and practical methods applied in many oilfields (Vazquez et al. 2016a).
2.1.3.2 Squeeze lifetimes
The squeeze lifetime is reflected by the cumulative produced water volume whose inhibitor concentration is above the MIC. The greater the volume of cumulative produced water above the MIC the longer the squeeze lifetime and vice versa. The squeeze lifetime may depend on several factors including retention/release properties of the reservoir formation, over-flush volume, amount of inhibitor and the MIC. A reservoir formation with high absorptive capacity retains a high amount of inhibitor, thereby prolonging the squeeze lifetime. A large over-flush volume is beneficial for optimizing squeeze lifetime. When a large over-flush volume is used the inhibitor slug is diluted causing deeper penetration of the scale inhibitor. This makes the reservoir formation retain/absorb more inhibitor. Moreover, the amount of scale inhibitor deployed and the MIC influence the squeeze lifetime. More inhibitor will be retained in the reservoir formation following deployment of a large amount of inhibitor. The lower MIC helps the treatment to last longer as it will take time to drop to lower concentration levels. These factors are discussed in length elsewhere (Shuler 1993; Vazquez et al. 2016b).
2.1.3.3 Models for squeeze treatments
Optimization of squeezes and enhancement for the squeeze lifetimes at a minimum cost have been a foremost goal during adsorption or precipitation squeeze treatments (Vazquez et al. 2013). In attempts to achieve that many computerized models (from simple to complex) have been put forward to describe the squeeze processes. The model developed may incorporate the adsorption/desorption parameters (i.e., first and second isotherm, and desorption rate constant) and/or precipitation/dissolution parameters (i.e., solubility and dissolution rate constant). In addition, the model can incorporate mechanical transport parameters such as mechanical transport rate constant, reaction order for the mechanical transport, and the fraction of precipitated inhibitor subjected to the mechanical transport. Principally, the model may be formulated on basis of equilibrium and/or kinetic forms, detailed discussion, formulation, and applicability can be found elsewhere (Malandrino et al. 1995; Shuler 1993; Sorbie and Gdanski 2005; Vazquez et al. 2013).
Criteria for model selection for simulating scale inhibitor squeeze may rely on the anticipated squeeze mechanism(s), scale inhibitor type, actual field conditions and the operational parameters such as shut-in and over-flush volume. Evaluation of scale inhibitor under reservoir conditions is often done prior to optimization of squeeze design. Following that, the model is used to predict the squeeze lifetime and to optimize the squeeze design. Proposed squeeze outcomes may reflect the behavior of scale inhibitor squeeze and the squeeze lifetime in the actual field. Some models had been reported to describe the squeeze design poorly as they cannot integrate all reservoir and operational conditions, and the changes associated during operation. A general view is that a good model is one capable of depicting the interaction between scale inhibitor and rock formation regardless of a retention mechanism, i.e., adsorption/desorption or precipitation/dissolution (Sorbie and Gdanski 2005), also it should optimize squeeze treatments and their lifetimes at minimal costs. Figure 4 shows a representative comparison between simulated and actual field results, more research work can be found elsewhere (Jordan et al. 2008; Malandrino et al. 1995; Vazquez et al. 2016b, 2013).
A reprehensive of scale inhibitor model simulated and field return concentration match: credit (Vazquez et al. 2016a)
2.1.3.4 Challenges for squeeze treatment
One of the major challenges for downhole squeezing is desorption of the chemical inhibitor (Przybylinski 1989; Rabaioli and Lockhart 1996; Sorbie and Gdanski 2005). When the pressure applied down the well is reversed, about 30% of the chemical inhibitor is often immediately flushed from the rock due to weak retention. Despite the remaining chemical inhibitor adsorbed onto the rock surface acting to inhibit scale formation, the inhibitor is gradually washed from the rock surface as oil production continues until a further descaling treatment is required.
Changes in wettability and permeability of the rock formation are another challenge facing downhole squeeze treatment. The aqueous solutions of applied scale inhibitors tend to change the reservoir wettability. This could result in a change in water permeability of the rock and could lead to a so-called water coning effect, wherein a water channel could over time open up into a water pocket. The well may then be irreversibly damaged and will never be able to reach its optimal productivity again (Armenta and Wojtanowicz 2002; Hatzignatiou and Mohamed 1994; Okon et al. 2017). The precipitated inhibitors can cause clogging of pores or formation damage (Jordan et al. 1994, 1995; Przybylinski 1989), thus, reducing the permeability of the rock formation which can cause reduced fluid flow.
Another challenge is that the treatment method may be less efficient in some regions where hydrocarbon formations have low permeability, especially horizontal hydrocarbon well formations (James et al. 2005). Scale inhibitors which are Newtonian fluids have difficulties reaching into formations, thus, the squeeze treatment with such fluids is not efficient in these regions and may cause the deposition of scale which can then block these regions, resulting in decreased production rates.
Also, precipitation squeeze treatments are less effective in non-carbonate reservoirs. They have been proven very successful only in carbonate reservoirs, generally with a longer useful life than with conventional adsorption squeeze treatments. Non-carbonate reservoirs do not offer sufficient amounts of calcium ions to cause precipitation of scale inhibitors.
Moreover, squeeze treatments sometimes do not give ideal scale inhibitor return curves. In most cases, scale inhibitors initially desorb to give high concentrations of scale inhibitors in the produced waters, which are usually much greater than that required to prevent scale formation. Thus, the concentration of scale inhibitor tends to decrease until it eventually falls below MIC. Hence, the oil well is at risk and needs to have retreated; regular retreatments of the well are highly discouraged as oil production always needs to be stopped to allow the treatments to be carried out.
2.1.3.5 Advancements on scale inhibitor treatments
As the conventional techniques met challenges for effectiveness, many studies were conducted for more advanced techniques (James et al. 2005; Jordan et al. 1995; Khormali et al. 2017; Sutherland and Jordan 2016; Vazquez et al. 2012). Thus, better regarded treatment methods that do not suffer from the disadvantages that beset conventional techniques were suggested. In advanced scale treatments, scale inhibitor systems integrate scale inhibitor and fracture treatments into a single step, which ensures that the entire well is treated with scale inhibitor. In this method, a high-efficiency scale inhibitor is pumped into the matrix surrounding the fracture face during leak off. The scale inhibitor is said to adsorb onto the matrix during pumping until the fracture begins to produce water. Produced water is anticipated to desorb sufficient scale inhibitor on passing through the inhibitor-adsorbed zone so the dissolved inhibitor prevents scale deposition. In this type of treatment, the scale inhibitor is better placed than in a conventional squeeze treatment, thereby lowering scale inhibitor leaching; hence, it reduces the retreatment cost and improves production.
In order to achieve an effective scale inhibitor treatment for a rock formation with low permeability, the use of fully viscosified scale squeeze fluids has been suggested in order to optimize the squeeze treatment by allowing the fluid to reach the low permeability region and the horizontal zones (James et al. 2005).
Moreover, the use of precipitation squeezes in non-carbonate reservoirs is suggested to provide the same extended life treatment as in carbonate reservoirs. To achieve such a squeeze in non-carbonate (or sandstone) reservoirs, both metal ions and scale inhibitor must be included in the solution placed in the reservoir. The added metal ions are expected to undergo precipitation reactions with the scale inhibitor such as phosphonates, thus the scale inhibitor lifetime is increased.
2.2 The key properties of scale inhibitors
For many years, scale inhibitors have been preferred for downhole treatment for controlling scale deposition (Vetter 1976, 1973). Many factors are usually considered for a chemical compound to be suitable as a scale inhibitor in the industry. The main five properties for a compound to suit as a scale inhibitor for squeeze treatments are as follows (Graham et al. 1998; Sanders et al. 2014):
-
1.
Compatibility The chemical should be compatible with the field brines and other chemical additives for enhanced oil recovery (EOR).
-
2.
Threshold inhibition The chemical should be capable of inhibiting scale formation at very low concentrations, typically of the order of 1–50 ppm.
-
3.
Long “squeeze” lifetime The chemical should show a long return profile from the reservoir (typically 3–12 months) at levels above the required threshold level or MIC. For economic reasons, an effective “squeeze” treatment chemical scale inhibitor should not merely be capable of inhibiting scale, but also should bond strongly with the formation to provide sufficiently extended return curves. If the scale inhibitor does not adsorb strongly enough it will all leach out very quickly and the oil well will require retreatment after a short time.
-
4.
Thermally stable The chemical should be relatively thermally stable under working conditions. It should not undergo thermal degradation under downhole conditions.
-
5.
Corrosion In the petroleum industry, scaling process rarely occurs in environments where no corrosion exists (Sanders et al. 2014). Thus, the scale inhibitor should neither trigger corrosion nor affected by corrosion products. Meanwhile, it should be compatible with the corrosion inhibitors (Spicka et al. 2011).
2.3 Scale inhibition mechanisms
The inhibition mechanism by which a scale inhibitor functions depends on its chemical nature, whether is a chelating (or sequestering) agent or threshold scale inhibitor. It can function by one or more mechanisms as described below. A chemical scale inhibitor can also function as a surface conditioner to preventing scaling. In this way, the tubing walls or equipment surfaces are conditioned in such a way that the adherence of crystals onto the walls or surfaces is prevented.
2.3.1 Chelants (sequestrants)
Chelating agents function by sequestration/chelation (binding cations to form stable water-soluble complexes) on preventing scale formation. The negative parts of the scale inhibitor molecules attract the metal cations in the solution, thereby forming coordinate bonds with the scaling cations (Fig. 5). In so doing, the cation is prevented from reacting with scaling anions in the solution; hence, the scaling process is prevented. The chelant molecules will bond with as many scaling cations as possible depending on the stoichiometric ratio. The stronger the coordinate bond between the scaling cation and the negative part of scale inhibitor species the better the inhibition. Chelants will prevent the scaling process only for a certain limited level of oversaturation, but if the equilibrium system is upset the precipitation begins.
A representation of an EDTA chelating molecule, as presented by Crabtree et al. (1999)
Examples of common chelants are ethylenediaminetetraacetic acid (EDTA), diethylenetriaminepentaacetic acid (DTPA), citric acid, and gluconic acid. Prevention of scale formation by using chelating agents is costly. A large amount of the chemical is needed for successful inhibition as they function in a stoichiometric manner. Thus, they are usually applied in the field as chemical dissolvers for removing mineral scale deposits (Kumar et al. 2018; Olajire 2015).
2.3.2 Threshold scale inhibitors
Threshold scale inhibitors prevent scale precipitation by delaying or preventing the mineral crystal nucleation and/or crystal growth. They prevent or retard the scale deposition by intervening in one (or more) step(s) of scale formation (i.e., intervening in aggregation, nucleation, crystal growth and/or agglomeration). They are believed to function primarily in one or a combination of threshold inhibition, nucleation inhibition, crystal distortion, and/or dispersion mechanisms (Abdel-Aal and Sawada 2003; Crabtree et al. 1999; Issabayev et al. 2018). Threshold inhibition refers to an inhibition mechanism by which a substoichiometric amount of inhibitor retards the crystal growth or delays the precipitation. In this mechanism, the scale inhibitor interacts with growing scale crystals; the scale inhibitor acts as a nucleation center that covers the scale formed in the aggregate. Thus, active crystal growth sites are blocked and further crystal growth is stopped.
Nucleation inhibition involves the disruption/redissolving of the growing nuclei (Sorbie and Laing 2004; Tomson et al. 2003). The inhibitor endothermically adsorbs onto growing embryo crystals causing their dissolution. In this mechanism, the thermodynamic stability of the formed nuclei is disrupted in the presence of the chemical scale inhibitor causing dissolution of embryos (Tung et al. 2004). The chemical inhibitors that function in this mode are termed as nucleation inhibitors. Organic polymers and phosphino-polycarboxylic acid (PPCA) scale inhibitors usually function in this mode (Kumar et al. 2018; Zhang et al. 2016b).
Crystal distortion (or retardation or modification) refers to the inhibition process whereby the scale inhibitor distorts the orderly growth of scale crystals causing them to be deformed and physically weak (Benton et al. 1993; Sorbie and Laing 2004; Tomson et al. 2003; Yuan et al. 1998). In this mechanism, a scale inhibitor is believed to interfere with the nucleation process or rate by adsorbing onto the active sites of scale crystals and distorting crystal morphology. Thus, the formation of a regular morphology and crystalline lattice and the buildup of an adherent scale are prevented. Adsorption of scale inhibitor on the active crystal growth sites can also slow the kinetics of crystal growth. In order to be effective, the scale inhibitor should be present during the nucleation stage of crystal growth. Chemical inhibitors that function by this mechanism are known as crystal modifiers (or crystal growth modifiers or crystal-modifying agents) (Benton et al. 1993). Examples of threshold scale inhibitors that mainly function in this mode are phosphonate inhibitors (Jones and Rohl 2005; Laing et al. 2003; Zhang et al. 2016b).
In the dispersion (or anti-agglomeration (Yuan et al. 1998)) mechanism, the negatively charged parts of a scale inhibitor adsorb onto scale micro-crystals. In so doing, they impart relatively high ionic charges that separate these particles and prevent them from agglomerating and settling as suspended particles. This prevents the crystal from adhering to a surface and growing into a scale deposit (i.e., particles are kept suspended in solution) (Hasson et al. 1998). The chemical inhibitors that function by this mechanism are known as dispersants. Examples of threshold scale inhibitors that mainly function in this mode are organic polymeric inhibitors (Zhang et al. 2016b).
2.4 Evaluation of scale inhibitors
Scale inhibitors are customarily evaluated in the laboratory prior to deployment into an actual field. The evaluation is aimed at choosing a suitable inhibitor candidate, which will effectively and economically prevent/retard scaling for an actual field. Numerous laboratory test methods have been suggested, Nonetheless, scale precipitation tests (static and dynamic) and core flood techniques are widely applied for screening and ranking scale inhibitors in the industry. The methods generally do not include all the likely kinetic, thermodynamic, and hydrodynamic conditions encountered by the inhibitor in the actual field (Baugh et al. 2012). This can lead to an impractical inhibition performance in some circumstances. A more informative discussion regarding the evaluation of scale inhibitors can be found elsewhere (Graham et al. 1998; Jordan et al. 1996).
2.4.1 Scale precipitation tests
These test methods are based on scale precipitation from supersaturated brines. Basically, two chemically incompatible brines (scaling cation-containing and anion-containing) are combined, resulting in precipitation under specified/variable conditions. One of the brine components (usually scaling anion-containing) is treated with varied inhibitor concentrations prior to mixing. In similar protocols, another run is done in the absence of inhibitor for comparison, and the inhibition performance is evaluated. The results are useful in establishing the minimum inhibition concentration (MIC) of the inhibitor and providing information regarding the inhibitor’s thermal stability (Jordan et al. 1996). The tests may provide information regarding inhibition mechanisms in addition to other techniques such as scanning electron microscopy (SEM) and X-ray powder diffraction (XRD). Depending on the experimental conditions employed, the method is differentiated into static or dynamic tests (Azizi et al. 2018).
Static scale precipitation test: This involves mixing up two chemically incompatible brines, giving a higher supersaturation index under static conditions. It is also known as “static bottle test” or “static inhibition efficiency test.” Synthetic brines, sometimes actual brines from the field, are mixed at defined proportions and pH values in the presence and absence of inhibitor. Mixed test brines are usually incubated at a constant temperature for definite time intervals (customarily no longer than 24 h). The supernatants are analyzed after the definite time intervals, and the inhibitor’s effectiveness is determined by its ability to retain scaling ions in solution compared to untreated brines. Many methods have been employed for analyzing ions in the supernatants: titrimetric, spectrometry, calorimetric, electrical conductivity (Azizi et al. 2018), light scattering (Sousa and Bertran 2014), pH measurement techniques (Sousa et al. 2016) and the like. Concurrently, a considerable number of protocols is known for carrying out the static bottle test. The NACE Standard Testing Methods (TM0374 and TM0197) and Chinese Petroleum Industry Standard SY/T 5673-1993 are among notable protocols for static bottle tests in the industry.
The static bottle test gives information about inhibitor’s effectiveness and thermal stability in preventing general scale formation in the bulk phase and/or bottle surfaces. The results may be used to predict inhibition mechanisms in assistance with other techniques. The method is predominant for preliminary evaluation because it is a quick test, cost-effective (as it does not require costly equipment), and does not necessarily require an expert to run (Baugh et al. 2012). Unfortunately, the method is less accurate for water with very high concentrations of scaling ions and can present less reproducible results (typically for carbonate scales due to inappropriate pressure control). Moreover, the bottle tests cannot exceed a testing temperature of about 95 °C (Jordan et al. 1996).
Dynamic scale precipitation test It is sometimes called “dynamic tube blocking test.” Under this method, the scaling cation-containing and anion-containing brines are pumped separately at a specified temperature and pressure, allowing brines to mix and flow via a coiled capillary tube. Principally, any scale deposition in the test coil will decrease the bore size of the coil, causing an increased pumping pressure, which is detected by a differential pressure transmitter. The differential pressure will increase with an increase in the scale deposit reaching to a point where the system is judged to be blocked, causing the apparatus to automatically shut down. The length of time taken for the coil to block in the presence and absence of the scale inhibitor is noted and used for determining the inhibitor’s effectiveness.
Unlike the static bottle tests, the dynamic tube blocking test gives information about inhibitor’s effectiveness in preventing scale buildup in a capillary tube using a flowing system. The method allows variations in thermodynamic and hydrodynamic test conditions and can be conducted in the presence of dissolved gases such as CO2 (Graham et al. 1998; Jordan et al. 1996). The method widely used in the industry as it presents an advantage of reflecting field conditions and better accounting for the adherence of scale to pipework. Nevertheless, the method is more expensive and more complex, requiring an expert to run.
2.4.2 Core flood
Unlike scale precipitation tests, core flood tests are aimed at evaluating the performance of scale inhibitors in the reservoir substrate (Graham et al. 2001). They provide information about the potential formation damage due to scale inhibitor treatment (Azizi et al. 2018; Khormali et al. 2018). In combination with tube blocking tests, the results are useful for prediction of potential return lifetimes under the test conditions. Tests can be performed from simple core flooding at simple conditions to full reservoir conditions including crude oil depending on the equipment design used. The tests provide vital data for optimizing scale inhibitor deployment; however, they are expensive.
3 Threshold scale inhibitors (TSIs)
A wide range of oilfield scale inhibitors nowadays are synthetic (man-made) chemical inhibitors and usually “threshold” scale inhibitors. In contrast to chelants, “threshold” scale inhibitors interact chemically with crystal nucleation sites and substantially reduce crystal growth rates (Abdel-Aal and Sawada 2003; Crabtree et al. 1999; Gao et al. 2015; Li et al. 2016; Sorbie and Laing 2004; Vazquez et al. 2016a). They are cost-effective because they can effectively prevent scale formation at low concentrations (i.e., non-stoichiometric), usually on the order of 1000 times less than a balanced stoichiometric ratio.
3.1 Classes of TSIs
Although a wide variety of mineral scale inhibiting chemicals is known at present, the threshold scale inhibitors most commonly used in oil well treatments are phosphorous-containing compounds, polycarboxylates or sulfonated compounds. They can be classified into three groups; inorganic phosphates, organophosphorus, and organic polymers (Abdel-Aal and Sawada 2003; Amjad et al. 2014; Gao et al. 2015; Issabayev et al. 2018; Olajire 2015; Wang et al. 2018).
3.1.1 Inorganic phosphates
The inorganic phosphate scale inhibitors which have been in use for many years are polyphosphates (Issabayev et al. 2018; King 1947), usually condensation polymers of orthophosphates. They are known in the petroleum industry as both scale and corrosion inhibitors (Abd-El-Khalek and Abd-El-Nabey 2013; Cohen 1946; Moudgil et al. 2009). The two commercially available phosphate scale inhibitors are sodium triphosphate and sodium hexametaphosphate (Fig. 6), and the frequent dosage is about 2–5 mg/L and 1–5 mg/L (Abd-El-Khalek and Abd-El-Nabey 2013), respectively. The maximum dosage usually is about 5 ppm due to limited solubility in brines. When the dosage is higher there is re-precipitation of polyphosphates instead of producing better inhibition effects.
Polyphosphates are very effective scale inhibitors; however, their application can be limited by low solubility and lower thermal stabilities than the phosphonate scale inhibitors (Issabayev et al. 2018; Wang et al. 2017). Their inhibitory properties are significantly affected by rising temperature. Also, they hydrolyze into orthophosphates with the formation of insoluble calcium salts (Wang et al. 2017; Zhang et al. 2010). Despite their limitations, polyphosphates are advantageous in that they can inhibit corrosion by forming a protective layer on the surface of the metal under certain conditions.
3.1.2 Organophosphorus
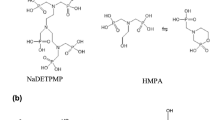
This includes all forms of phosphorous-containing organic scale inhibitors. They can be organic phosphonates (i.e., organophosphates) or organic phosphate esters. Organic phosphonates include organic phosphonic acids and the salts thereof; and can be nitrogen-containing organic phosphonates (i.e., amino-alkylene phosphonates), Fig. 7. The commonly commercially available organic phosphonate scale inhibitors include amino-alkylene phosphonic compounds (Moudgil et al. 2009; Sorbie and Laing 2004; Zhang et al. 2010) such as aminotrimethylene phosphonic acid (ATMP), ethylenediaminetetramethylene phosphonic acid (EDTMP), diethylenetriaminepentamethylene phosphonic acid (DETPMP), and pentaethylenehexamineoctakismethylene phosphonic acid (PEHOMP) to mention few. Other phosphonates are non-nitrogen-containing phosphonic compounds such as 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) (Amjad et al. 2014; Yang et al. 2017), polymeric compounds from vinylphosphonic acid, for example, polyvinyl phosphonic acid (PPA). Moreover, phosphonate scale inhibitors also include phosphino-carboxylic acids (PCA) (Martinod et al. 2008; Sorbie and Laing 2004) such as poly(phosphino-acrylic acid) (PPCA), as well as sulfonated phosphino-carboxylic acid (SPOCA) and their derivatives. Apart from organic phosphate, organophosphorus scale inhibitors can be organic phosphate ester compounds (El Dahan and Hegazy 2000; Valiakhmetova et al. 2017).
Organic phosphonate scale inhibitors are advantageous in that they do not easily hydrolyze and can tolerate a wide range of temperatures, being thermally stable to 250 °F (121.1 °C) but can go higher in the absence of oxygen. They are low dosage (usually used at a dosage of 1–5 mg/L) and corrosion inhibitors as well (Browning and Fogler 1996). Most of them are good squeeze inhibitors as they have good adsorbance to the rock giving a longer squeeze lifetime. They have a high tolerance for calcium ions, thus are considered to be CaCO3 and CaSO4 scale inhibitors (Zhang et al. 2010). Despite those advantages, their applications are limited to environmental implications (Wang et al. 2014) and usually are relatively expensive due to the costly methods used to synthesize them. Also, in high calcium ion concentrations phosphonates react stoichiometrically with the calcium ions and form insoluble Ca–phosphonate precipitates which can be as detrimental as other scales (Browning and Fogler 1996; Zhang et al. 2010, 2016c). Nevertheless, phosphino-carboxylic acid and its modified types are potentially used as multifunctional inhibitors and they have advantages over unifunctional inhibitors in performance, dispersancy, thermal stability and environmental acceptability (Martinod et al. 2008).
3.1.3 Organic polymers
These include polycarboxylic acids and their derivatives that can be their salts or esters with low alcohols, usually lower alcohols such as methanol or ethanol. The well-known organic polymer scale inhibitors are polymers of maleic anhydride (or maleic acid) (Amjad and Koutsoukos 2014; von Bonin 1983; Fukumoto and Moriyama 1987) and acrylic acid (or methacrylic acid) (Rabaioli and Lockhart 1996; Reddy and Hoch 2001; Wang et al. 2018) and their derivatives. Maleic anhydride (or maleic acid) and acrylic acid (or methacrylic acid) can be homopolymerized to give polymaleic anhydride (or polymaleic acid) and polyacrylic acid (or polymethacrylic acid), respectively, as shown in Fig. 8.
It had been found that copolymers are more effective than homopolymers, thus, in most cases, they are copolymerized (sometimes terpolymerized) with other monomers such as acrylamide, vinyl sulfonic acid, sulfonated styrene, and itaconic acid (Masler and Amjad 1988; Wang et al. 2018); example in Fig. 9.
Other organic polymeric compounds which are useful in the oil and gas industry for scale prevention are polymers of epoxycarboxylic acids and that of amino acids. Polyepoxysuccinic acid (Huang et al. 2018; Sun et al. 2009) and polyaspartic acid (Liu et al. 2012a; Roweton et al. 1997; Yang et al. 2017), Fig. 10, and their derivatives are often used for scale prevention. Recent studies have been on polycarboxylate esters (Popuri et al. 2014; Zhao et al. 2016), and well-known polycarboxylate esters including polycitric ester (Popuri et al. 2014; Zhao et al. 2016). Polycarboxylate esters are often less effective when applied alone and are thermally less stable, limiting their applicability as scale inhibitors.
Like organophosphorus scale inhibitors, organic polymeric scale inhibitors have shown excellent properties in delaying, reducing and/or preventing scale deposition in oil and gas production (Amjad and Koutsoukos 2014; Liu et al. 2012a). Organic polymeric scale inhibitors are advantageous in that they are “green” scale inhibitors; their application does not severely damage the environment (Liu et al. 2017; Yang et al. 2017). Also, they have excellent solubility allowing them to be superior in preventing scale deposition for highly saline environments. There is still an advantage for organic polymeric scale inhibitors in that they are relatively cheap; their synthetic routes are usually economic.
3.1.4 Blending scale inhibitors
In some cases two different classes of inhibitors can be blended together usually giving a synergistic effect, better inhibition effect than a single inhibitor, such inhibitors are referred to as blended scale inhibitors. For example, organic polymers and phosphonate inhibitors can be blended together to offer synergistic behavior (Shaw and Sorbie 2014). The reason for the resulted synergistic behavior is predicted to be that different inhibitor classes act via different mechanisms; thus, the combination of these mechanisms results in better inhibition (Kumar et al. 2018; Shaw and Sorbie 2014). Shaw and Sorbie (Shaw and Sorbie 2014) researched synergetic properties due to blends of phosphonate and polymeric scale inhibitors. According to their study, only 5 (out of 34) scale inhibitor blends showed detrimental inhibition properties; most of the blends showed synergistically enhanced inhibition efficiencies. For an enhanced synergistic inhibition, the inhibitor concentration in the blend should not fall below the minimum inhibitor concentration of either component. Enhanced synergistic inhibition is achievable when a scale inhibitor blend of an equal ratio is used; nevertheless, it is contrary in some cases (Khormali et al. 2018). Moreover, the scale inhibitor blends whose components exhibit different inhibition mechanisms usually show synergistically enhanced inhibition efficiencies because of multiple inhibition mechanisms.
3.2 Common synthetic routes
Every class of scale inhibitors described above has its own method(s) of synthesis which can be simple or complex. Synthetic methods may differ depending on the precursors used for synthesis within the same class of inhibitors. Here, common laboratory (or industrial) synthetic methods are included just for review; the methods described are for widely applied scale inhibitors in each class.
3.2.1 Inorganic phosphates
The representative inorganic scale inhibitors to be discussed here is triphosphate inorganic salts; archetypically sodium triphosphate (STP) sometimes referred to sodium tripolyphosphate (STPP). Triphosphate salts are usually produced simply by heating a stoichiometric mixture of phosphate salts: di- and mono-metallic phosphate salts. When such a mixture is heated at elevated temperatures, usually about 250–600 °C (Ficner et al. 1981; King 1947) for sufficient time; the phosphate salts thermally condense into STP or STPP. For instance, sodium triphosphate is produced when a stoichiometric mixture of disodium phosphate, (Na2HPO4), and monosodium phosphate, (NaH2PO4) is heated at elevated temperatures. One molecule of monosodium phosphate is thought to react with two molecules of disodium phosphate producing triphosphate with the liberation of two water molecules.
There are two consecutive steps for the reaction generating the targeted product. In the first step, disodium phosphate reacts with monosodium phosphate generating diphosphate as an intermediate with the liberation of one water molecule. In the subsequent step, another disodium phosphate reacts with the generated intermediate forming the product (sodium triphosphate) with the liberation of another water molecule. The overall reaction is simply represented as:
It is possible for the intermediate to react with monosodium phosphate forming triphosphate which is capable of undergoing further reaction producing polymeric phosphate. Thus, the term tripolyphosphate is preferred over triphosphate, hence, sodium triphosphate and sodium tripolyphosphate are interchangeably used.
In the industrial process, the mixture of mono- and disodium phosphate is often obtained by reacting a calculated amount of soda ash (Na2CO3) or caustic soda (NaOH) with phosphoric acid (Ficner et al. 1981; Kearns 1969; Lawrence 1984). When sodium carbonate reacts with phosphoric acid at a 2.5:3 mole ratio, it gives a salt mixture containing the monosodium phosphate and disodium phosphate having a 1:2 mol ratio. If caustic soda is used, the equivalent monosodium phosphate and disodium phosphate in a mole ratio of 1:2 is obtained when caustic soda and phosphoric acid reacts in a ratio of 5:3. In all reactions involving the formation of monosodium phosphate and disodium phosphate; the desired mole ratio for reacting phosphate salts is achieved when sodium and phosphorous for starting materials are in a mole ratio of 1.67:1 or 5:3 (Lawrence 1984), the reactions are as follows.
The phosphate salt mixture is further heated at elevated temperatures as described before to produce tripolyphosphate salts.
3.2.2 Organophosphorus compounds
Organophosphorus compounds widely used as scale inhibitors are amino-alkylene phosphonic compounds. The synthetic route for those compounds, in particular EDTMP, is described as an example. Also, preparation of poly(phosphino-carboxylic) acid and its modified types is highlighted.
3.2.2.1 Amino-alkylene phosphonic acids
Amino-alkylene phosphonic acids share common synthetic methods. The method of producing amino-alkylene phosphonic acids has been extensively discussed. They are widely produced in industry or the laboratory by reacting a nitrogen-containing compound such as amine or ammonia/ammonium salt or amide, methanal (formaldehyde) and a phosphorous(III) containing compound such as phosphorus trihalide, phosphorous acid or phosphorus oxides (Issabayev et al. 2018). In those processes, the nitrogen-containing compound such as the amine reacts first with formaldehyde generating an intermediate which then reacts with phosphorous acid with the application of heat. The reaction can be generally represented as:

In particular EDTMP, as an example of the said acids, is prepared by reacting ethylenediamine with formaldehyde and phosphorous acid at a temperature of about 110 °C. When such mixture is refluxed at about 110 °C often for about 3 h, EDTMP is formed. The reaction proceeding is represented as:

The above reaction results in the formation of a mixed product, i.e., a mixture of amino- alkylene phosphonic acids if the reagents are not supplied in balanced stoichiometric ratios. To overcome this, the use of excess phosphorous acid may result in the formation of purer products. Phosphorous acid can be replaced with inexpensive phosphorous trichloride or a mixture of phosphorous acid and phosphorous trichloride in the process of producing the said acids. Moreover, phosphorous oxides such as tetraphosphorous hexaoxide have been used to replace phosphorous acid. These oxides hydrolyze in an aqueous reaction medium containing a homogeneous Bronsted acid giving phosphorous acid.
3.2.2.2 Poly(phosphino-carboxylic) acids
Poly(phosphine-carboxylic) acid and its modified types are synthesized via free radical polymerization of unsaturated organic carboxylates with hypophosphorous compounds/acid (Richardson et al. 1987; Wang et al. 2014). The reaction is normally carried out in an inert solvent under specific reaction conditions and in the presence of a suitable initiator (free radical generating species). Richardson et al. (1987) patented and disclosed in the US Patent 4681686 the production of these compounds. They prepared the compounds by polymerizing unsaturated carboxylates and acrylamide-based monomers with hypophosphite salt. Inert solvents proposed are water, ethanol or dioxane. Suggested initiators include bisazoisobutyronitrile, organic peroxides such as benzoyl peroxide, methyl ethyl ketone peroxide, ditertiary butyl peroxide, monobutyl hydroperoxide, and oxidizing agents such as hydrogen peroxide, sodium perborate, sodium persulfate, potassium persulfate, and ammonium persulfate. The reactions are carried at refluxing temperatures.
3.2.3 Organic polymers
Organic polymeric scale inhibitors are commonly produced either via chain-growth or step-growth polymerization methods. The synthesis of maleic anhydride-based polymers and polyaspartic acid will be discussed as examples of the two methods.
3.2.3.1 Maleic anhydride-based polymers
Scale inhibitors based on maleic anhydride polymers and their copolymers are produced via free radical polymerization of unsaturated monomers using an initiator with the application of heat (Davies et al. 2005; Goretta and Newkirk 1976; Nasirtabrizi et al. 2013; von Bonin 1983). The polymerization is carried out under a nitrogen atmosphere using dry inert solvents such as benzene, toluene, xylene, ether, ethyl acetate, acetone, tetrahydrofuran and the like. Also, the reaction can be carried out in air (i.e., without nitrogen atmosphere). The reaction initiator used is one of the organic peroxides which generate free radicals under application of heat. For instance, it may be benzoyl peroxide (BPO), tert-butyl peroxybenzoate (TBPB), dicumyl peroxide (DCP), tert-butyl hydroperoxide (tBuOOH), di-tert butylperoxide (DTBP), azobisisobutyronitrile (AIBN) and the like. The amount of peroxide initiator may vary depending on the reaction conditions and solvent used, usually from about 0.1% to 10% by weight based on the monomers. The reaction temperature varies from 60 °C to about 200 °C (Deb and Meyerhoff 1985; von Bonin 1983), commonly near the reflux temperature of the solvent used. The polymeric product produced by this synthetic route can be poly(maleic anhydride) or copolymer or terpolymer of maleic anhydride. The product can be hydrolyzed giving the corresponding maleate (or salt) polymeric product.
On the other hand, polymerization of maleic anhydride and its polymerizable monomers may be carried in aqueous solution (Fukumoto and Moriyama 1987). The polymerization process is usually carried out using inorganic peroxide compounds that generate free radicals to initiate the reaction. Inorganic peroxides used may include persulfate compounds such as sodium persulfate (Na2S2O8), potassium persulfate (K2S2O8), ammonium persulfate ((NH4)2S2O8) or perhaps hydrogen peroxide (H2O2). The polymeric product obtained is usually polymaleate or copolymer or terpolymer of maleate. It is extremely difficult for maleic acid to undergo both homopolymerization and copolymerization due to the severe steric hindrance and polar effects of two carboxylic acid groups. Thus, polymers of maleic acids are conventionally prepared by hydrolyzing corresponding maleic anhydride polymers.
3.2.3.2 Polyaspartic acid (PASP)
PASP is one of the commonly applied scale inhibitors. It has been widely applied in the oil and gas industry due to its good environmental and inhibition performance (Ajikumar et al. 2005; Migahed et al. 2016; Quan et al. 2008; Thombre and Sarwade 2005). It is a non-phosphorous biodegradable polymer, showing excellence performance on CaSO4 and CaCO3 scales. PASP has been widely produced by several methods with and without catalyst (Liu et al. 2011; Roweton et al. 1997; Thombre and Sarwade 2005). In general, many methods involve the formation of polysuccinimide (PSI) which can be hydrolyzed to acid or salt form.
The direct synthetic route for PASP involves thermal polycondensation of d- and l-aspartic acid. The polymerization process can be carried out in the absence or presence of an acid catalyst such as phosphoric acid at relatively high temperatures to about 300 °C (Bennett 2005; Gao et al. 2015; Liu et al. 2011; Wang et al. 2003). In this method, there are two steps involved which both result in a loss of water molecules (condensate). The first step might be the loss of one water molecule via the reaction between a hydrogen of an amino group of one aspartic acid molecule and a hydroxyl of the carboxyl group of another aspartic acid molecule, forming amide bonds. In the second step, the water molecule can be lost as a result of the reaction between amide hydrogen and another hydroxyl group, forming the succinimide ring. The two steps result in the formation of PSI which is subsequently hydrolyzed to give poly(aspartate), as shown in Fig. 11.
It had been found that acid catalyzed polymerization of aspartic acid into PSI is substantially faster and gives a polymeric product with higher molecular weight than uncatalyzed polymerization (Wang et al. 2003).
PASP also had been produced via thermal polycondensation processes that involve the use of maleic anhydride or its derivatives. Such methods are relatively inexpensive and require less energy. Thermal polycondensation of maleic anhydride and ammonium carbonate (Liu et al. 2011) or thermal polycondensation of ammonium maleate salt (Zhang et al. 2016d) are also common methods for producing PASP. Both methods proceed via the formation of PSI which is then hydrolyzed into PASP.
4 Environmental concerns about synthetic scale inhibitors
Many of synthetic scale inhibitors are toxic and/or non-biodegradable; their disposal may pollute the environment and harm living organisms, so environmental concerns and discharge limitations have increased nowadays. These considerations have increasingly prompted synthetic SIs chemistry to move toward “green” scale inhibitors which are readily biodegradable with low mobility posing minimum environmental implications (Liu et al. 2017, 2016; Wang et al. 2017; Yang et al. 2017; Zhao et al. 2016).
The petroleum industry currently has been under severe restrictions on the discharge of oilfield chemicals into the environment (Lattemann and Höpner 2008). The restrictions have prompted the industry to assume rational management of industrial wastes, and it has been implementing more restrictive steps concerning their disposal. Obtaining cost-effective and environmental-friendly scale inhibitors with low effective dosages has been of great interest for many researchers (Chaussemier et al. 2015; Popuri et al. 2014; Reis et al. 2012). The industry is trying to limit some applications of conventional phosphorous scale inhibitors, replacing them with less organic phosphorus compounds. Moreover, the industry has been encouraging the use of new classes of less toxic compounds based on non-phosphorus chemistry (Martinod et al. 2008). Polymeric scale inhibitors such as polymaleates, polyacrylates, and derivatives thereof (sulfonated homo- and copolymers) have been gaining attention in the petroleum industry.
Along with the aforementioned considerations, interest has turned to naturally occurring scale inhibitors (Chaussemier et al. 2015; Menzri et al. 2017; Reno and Endaryanto 2017). Modified natural plant extracts such as insulin-modified compounds have been studied for their applications as scale inhibitors (Kırboga and Öner 2012; Rahul et al. 2014; Zhang et al. 2016a). Also, natural products that contain substantial amounts of polyphosphates, carboxylic acid groups, alcohol, and aromatic amines with potential functionalities for adsorption or anti-scaling effect have considered (Abdel-Gaber et al. 2011; Chaussemier et al. 2015; Khamis et al. 2018). Such extracts and their derivatives are environmentally friendly, non-toxic, low bioaccumulation, biodegradable, relatively less expensive, readily and sustainably available. They are generally considered as the greenest scale inhibitors. However, the biodegradability factors of those materials limit their storages and long-term applications.
5 Conclusion and remarks
Through this review, it is concluded that threshold scale inhibitors are cost-effective and convenient for preventing scaling in the petroleum industry and other areas where mineral scaling is likely. The effectiveness depends on several factors such as water chemistry, temperature, and pressure. Any factor that will reduce the adsorption/binding potential of the threshold scale inhibitors will reduce their effectiveness. In some circumstances (for example, where the scaling potential is extremely high) threshold scale inhibitors are unable to prevent scale formation. The solubility of the threshold scale inhibitor is also important for its effectiveness. Its solubility should be reasonable so it dissolves to give an adequate concentration that will effectively inhibit scale formation.
Scale inhibitors should be evaluated before being deployed in the field. They may cause problems such as formation damage, increased corrosion, and sometimes deposition of more scales. Phosphonate inhibitors normally exhibit lower MIC and better thermal stability than most of the organic polymers. The effectiveness of the scale inhibitor may increase when two or more scale inhibitors are blended. The combination may provide a synergistic result (better performance than a simple additive effect). Blending should be carefully done as it may also cause poorer results than the predicted additive effect.
A method for scale inhibitor deployment should be one which will optimize the inhibition performance and chemical usage and will increase the inhibition lifetime as well. The type of inhibitor and the physiochemical properties of the reservoir formation play a vital role in deciding the deployment method.
Moreover, because of increased environmental concerns, threshold scale inhibitors designed for use should preferably exhibit acceptable biodegradation properties with low toxicity and low bioaccumulation.
References
Abd-El-Khalek DE, Abd-El-Nabey BA. Evaluation of sodium hexametaphosphate as scale and corrosion inhibitor in cooling water using electrochemical techniques. Desalination. 2013;311:227–33. https://doi.org/10.1016/J.DESAL.2012.11.017.
Abdel-Aal N, Sawada K. Inhibition of adhesion and precipitation of CaCO3 by aminopolyphosphonate. J Cryst Growth. 2003;256:188–200. https://doi.org/10.1016/S0022-0248(03)01354-X.
Abdel-Gaber AM, Abd-El-Nabey BA, Khamis E, Abd-El-Khalek DE. A natural extract as scale and corrosion inhibitor for steel surface in brine solution. Desalination. 2011;278:337–42. https://doi.org/10.1016/J.DESAL.2011.05.048.
Ajikumar PK, Low BJM, Valiyaveettil S. Role of soluble polymers on the preparation of functional thin films of calcium carbonate. Surf Coat Technol. 2005;198:227–30. https://doi.org/10.1016/J.SURFCOAT.2004.10.028.
Al-Roomi YM, Hussain KF. Potential kinetic model for scaling and scale inhibition mechanism. Desalination. 2016;393:186–95. https://doi.org/10.1016/J.DESAL.2015.07.025.
Amiri M, Moghadasi J. The effect of temperature, pressure, and mixing ratio of injection water with formation water on barium sulfate scale formation in Siri Oilfield. Energy Sour A Recover Util Environ Eff. 2013;35:1316–27. https://doi.org/10.1080/15567036.2010.516322.
Amjad Z, Demadis KD (eds). Patent review related to scale and scale inhibition. In: Mineral scales and deposits. Elsevier; 2015. p. 239–319. https://doi.org/10.1016/B978-0-444-63228-9.00011-5.
Amjad Z, Koutsoukos PG. Evaluation of maleic acid based polymers as scale inhibitors and dispersants for industrial water applications. Desalination. 2014;335:55–63. https://doi.org/10.1016/J.DESAL.2013.12.012.
Amjad Z, Landgraf RT, Penn JL. Calcium sulfate dihydrate (gypsum) scale inhibition by PAA, PAPEMP, and PAA/PAPEMP blend. Int J Corros Scale Inhib. 2014;3:35–47. https://doi.org/10.17675/2305-6894-2014-3-1-035-047.
Andrei M, Malandrino A. Comparative coreflood studies for precipitation and adsorption squeeze with PPCA as the scale inhibitor. Pet Sci Technol. 2003;21:1295–315. https://doi.org/10.1081/LFT-120018174.
Armenta M, Wojtanowicz A. Severity of water coning in gas wells. In: SPE gas technology symposium, April 30–May 2, Calgary, Alberta; 2002. https://doi.org/10.2118/75720-MS.
Azizi J, Shadizadeh SR, Manshad KA, Mohammadi AH. A dynamic method for experimental assessment of scale inhibitor efficiency in oil recovery process by water flooding. Petroleum. 2018. https://doi.org/10.1016/J.PETLM.2018.07.004.
Baugh TD, Lee J, Winters K, Waters J, Wilcher J. A fast and information-rich test method for scale inhibitor performance. In: Offshore technology conference, 30 April–3 May, Houston, Texas; 2012. https://doi.org/10.4043/23150-MS.
Bennett GD. A green polymerization of aspartic acid for the undergraduate organic laboratory. J Chem Educ. 2005;82(9):1380–1. https://doi.org/10.1021/ed082p1380.
Benton WJ, Collins IR, Grimsey IM, Parkinson GM, Rodger SA. Nucleation, growth and inhibition of barium sulfate-controlled modification with organic and inorganic additives. Faraday Discuss. 1993;95:281–97. https://doi.org/10.1039/fd9939500281.
Browning FH, Fogler HS. Effect of precipitating conditions on the formation of calcium—HEDP precipitates. Langmuir. 1996;12(21):5231–8. https://doi.org/10.1021/LA9603277.
Chauhan K, Sharma P, Chauhan GS. Removal/dissolution of mineral scale deposits. In: Amjad Z, Demadis KD, editors. Mineral scales and deposits. Elsevier; 2015. p. 701–20. https://doi.org/10.1016/B978-0-444-63228-9.00029-2.
Chaussemier M, Pourmohtasham E, Gelus D, Pécoul N, Perrot H, Lédion J, et al. State of art of natural inhibitors of calcium carbonate scaling: a review article. Desalination. 2015;356:47–55. https://doi.org/10.1016/J.DESAL.2014.10.014.
Cohen M. Sodium hexametaphosphate as a corrosion inhibitor for Ottawa tap water. Trans Electrochem Soc. 1946;89(1):105–25. https://doi.org/10.1149/1.3071701.
Crabtree M, Eslinger D, Fletcher P, Miller M, Johnson A, King G. Fighting scale—removal and prevention. Oilf Rev. 1999;11(3):30–45.
Davies MC, Dawkins JV, Hourston DJ. Radical copolymerization of maleic anhydride and substituted styrenes by reversible addition-fragmentation chain transfer (RAFT) polymerization. Polymer (Guildf). 2005;46(6):1739–53. https://doi.org/10.1016/J.POLYMER.2004.12.037.
Deb PC, Meyerhoff G. Study on kinetics of copolymerization of styrene and maleic anhydride in methyl ethyl ketone. Polymer (Guildf). 1985;26(4):629–35. https://doi.org/10.1016/0032-3861(85)90166-1.
Dyer S, Graham G. The effect of temperature and pressure on oilfield scale formation. J Pet Sci Eng. 2002;35(1–2):95–107. https://doi.org/10.1016/S0920-4105(02)00217-6.
El Dahan HA, Hegazy HS. Gypsum scale control by phosphate ester. Desalination. 2000;127(2):111–8. https://doi.org/10.1016/S0011-9164(99)00196-4.
Ficner SA, Klanica AJ, Korenowski TF. Process for making sodium tripolyphosphate from wet process phosphoric acid. U.S. Patent No. 4251491. 17 Feb. 1981.
Fukumoto Y, Moriyama M. Production of polymaleic acid. U.S. Patent No. 4,709,091. 24 Nov. 1987.
Gao Y, Fan L, Ward L, Liu Z. Synthesis of polyaspartic acid derivative and evaluation of its corrosion and scale inhibition performance in seawater utilization. Desalination. 2015;365:220–6. https://doi.org/10.1016/J.DESAL.2015.03.006.
García AV, Thomsen K, Stenby EH. Prediction of mineral scale formation in geothermal and oilfield operations using the extended UNIQUAC model: Part I. Sulfate scaling minerals. Geothermics. 2005;34(1):61–97. https://doi.org/10.1016/J.GEOTHERMICS.2004.11.002.
Goretta LA, Newkirk JD. Polymerization of maleic anhydride and vinyl acetate. U.S. Patent 3,933,763. 20 Jan. 1976.
Graham GM, Dyer SJ, Shone P, Mackay EJ, Juhasz A. High temperature core flooding experiments for the selection of appropriate scale inhibitor products for potential application as downhole squeeze treatments in high temperature reservoir environments. In: International symposium on oilfield scale, January 30–31, Aberdeen; 2001. https://doi.org/10.2118/68314-MS.
Graham GM, Dyer SJ, Sorbie KS, Sablerolle WR, Shone P, Frigo D. Scale inhibitor selection for continuous and downhole squeeze application in HP/HT conditions. In: SPE annual technical conference and exhibition, September 27–30, New Orleans, Louisiana; 1998. https://doi.org/10.2118/49197-MS.
Hart PW, Rudie AW. Mineral scale management. Part 1: case studies. Tappi. 2006;5(6):22–7.
Hasson D, Semiat R. Scale control in saline and wastewater desalination. Isr J Chem. 2006;46(1):97–104. https://doi.org/10.1560/BM6M-01UJ-CNP2-W0E3.
Hasson D, Semiat R, Bramson D, Busch M, Limoni-Relis B. Suppression of CaCO3 scale deposition by anti-scalants. Desalination. 1998;118(1–3):285–96. https://doi.org/10.1016/S0011-9164(98)00149-0.
Hatzignatiou DG, Mohamed F. Water and gas coning in horizontal and vertical wells. In: Annual technical meeting, 12–15 June, Calgary, Alberta; 1994. https://doi.org/10.2118/94-26.
Huang H, Yao Q, Jiao Q, Liu B, Chen H. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J Saudi Chem Soc. 2018. https://doi.org/10.1016/J.JSCS.2018.04.003.
Issabayev YA, Boiko GI, Lyubchenko NP, Shaikhutdinov YM, Muhr H, Colombeau L, et al. Synthesis of unexplored aminophosphonic acid and evaluation as scale inhibitor for industrial water applications. J Water Process Eng. 2018;22:192–202. https://doi.org/10.1016/J.JWPE.2017.12.007.
Jamero J, Zarrouk SJ, Mroczek E. Mineral scaling in two-phase geothermal pipelines: two case studies. Geothermics. 2018;72:1–14. https://doi.org/10.1016/J.GEOTHERMICS.2017.10.015.
James JS, Frigo, DM, Heath SM, Graham GM, Townsend MM. Application of a fully viscosified scale squeeze for improved placement in horizontal wells. In: SPE international symposium on oilfield scale, May 11–12, Aberdeen; 2005. https://doi.org/10.2118/94593-MS.
Jonathan B. Production chemistry. Well Complet Des. 2009;371–432(56):371–432. https://doi.org/10.1016/S0376-7361(08)00207-0.
Jones F, Rohl AL. Empirical molecular modelling of crystal growth modifiers. Mol Simul. 2005;31(6–7):393–8. https://doi.org/10.1080/08927020412331333739.
Jordan MM, Mackay EJ, Vazquez O. The influence of overflush fluid type on scale squeeze life time: field examples and placement simulation evaluation. In: Corrosion, March 16–20, New Orleans, Louisiana; 2008.
Jordan MM, Sorbie KS, Graham GM, Taylor K, Hourston KE, Hennessey S. The correct selection and application methods for adsorption and precipitation scale inhibitors for squeeze treatments in North Sea Oilfields. In: SPE formation damage control symposium, February 14–15, Lafayette, Louisiana; 1996. https://doi.org/10.2118/31125-MS.
Jordan MM, Sorbie KS, Griffin P, Hennessey S, Hourston KE, Waterhouse P. Scale inhibitor adsorption/desorption vs. precipitation: the potential for extending squeeze life while minimising formation damage. In: SPE European formation damage conference, May 15–16, The Hague, Netherlands; 1995. https://doi.org/10.2118/30106-MS.
Jordan MM, Sorbie KS, Ping J, Yuan MD, Todd AC, Hourston KE. Phosphonate scale inhibitor adsorption/desorption and the potential for formation damage in reconditioned field core. In: SPE formation damage control symposium, February 7–10, Lafayette, Louisiana; 1994. https://doi.org/10.2118/27389-MS.
Kamal MS, Hussein I, Mahmoud M, Sultan AS, Saad MAS. Oilfield scale formation and chemical removal: a review. J Pet Sci Eng. 2018;171:127–39. https://doi.org/10.1016/J.PETROL.2018.07.037.
Kan AT, Fu G, Tomson MB. Adsorption and precipitation of an aminoalkylphosphonate onto calcite. J Colloid Interface Sci. 2005;281(2):275–84. https://doi.org/10.1016/j.jcis.2004.08.054.
Kearns TC. Manufacture of ammonium polyphosphate from wet process phosphoric acid. U.S. Patent No. 3,464,808. 2 Sep. 1969.
Khamis E, El-Rafey E, Abdel-Gaber A, El-Hefnawy A, El-Din MS. Arghel extract as an environmentally friendly anti-corrosion and anti-scalent in industrial water systems. IOP Conf Ser Mater Sci Eng. 2018;301(1):012149. https://doi.org/10.1088/1757-899X/301/1/012149.
Khormali A, Petrakov DG, Moghaddam NR. Study of adsorption/desorption properties of a new scale inhibitor package to prevent calcium carbonate formation during water injection in oil reservoirs. J Pet Sci Eng. 2017;153:257–67. https://doi.org/10.1016/j.petrol.2017.04.008.
Khormali A, Sharifov AR, Torba DI. Increasing efficiency of calcium sulfate scale prevention using a new mixture of phosphonate scale inhibitors during waterflooding. J Pet Sci Eng. 2018;164:245–58. https://doi.org/10.1016/J.PETROL.2018.01.055.
King CS. Manufacture of sodium triphosphates. U.S. Patent No. 2,419,148. 15 Apr. 1947.
Kırboga S, Öner M. The inhibitory effects of carboxymethyl inulin on the seeded growth of calcium carbonate. Colloids Surf B Biointerfaces. 2012;91:18–25. https://doi.org/10.1016/J.COLSURFB.2011.10.031.
Kodel KA, Andrade PF, Valença JVB, Souza DN. Study on the composition of mineral scales in oil wells. J Pet Sci Eng. 2012;81:1–6. https://doi.org/10.1016/J.PETROL.2011.12.007.
Kumar S, Naiya TK, Kumar T. Developments in oilfield scale handling towards green technology: a review. J Pet Sci Eng. 2018;169:428–44. https://doi.org/10.1016/j.petrol.2018.05.068.
Laing N, Graham GM, Dyer SJ. Barium sulphate inhibition in subsea systems—the impact of cold seabed temperatures on the performance of generically different scale inhibitor species. In: International symposium on oilfield chemistry, 5–7 February, Houston, Texas; 2003. https://doi.org/10.2118/80229-MS.
Lattemann S, Höpner T. Environmental impact and impact assessment of seawater desalination. Desalination. 2008;220(1–3):1–15. https://doi.org/10.1016/j.desal.2007.03.009.
Lawrence SA. Sodium tripolyphosphate manufacture. U.S. Patent No. 06,666,932. 31 Oct. 1984.
Li X, Shemer H, Hasson D, Semiat R. Characterization of the effectiveness of anti-scalants in suppressing scale deposition on a heated surface. Desalination. 2016;397:38–42. https://doi.org/10.1016/J.DESAL.2016.06.022.
Liu D, Dong W, Li F, Hui F, Lédion J. Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods. Desalination. 2012a;304:1–10. https://doi.org/10.1016/J.DESAL.2012.07.032.
Liu G, Xue M, Yang H. Polyether copolymer as an environmentally friendly scale and corrosion inhibitor in seawater. Desalination. 2017;419:133–40. https://doi.org/10.1016/j.desal.2017.06.017.
Liu W, Chien SH, Dzombak DA, Vidic RD. Mineral scaling mitigation in cooling systems using tertiary-treated municipal wastewater. Water Res. 2012b;46:4488–98. https://doi.org/10.1016/J.WATRES.2012.05.041.
Liu Y, Zou C, Li C, Lin L, Chen W. Evaluation of β-cyclodextrin–polyethylene glycol as green scale inhibitors for produced-water in shale gas well. Desalination. 2016;377:28–33. https://doi.org/10.1016/J.DESAL.2015.09.007.
Liu Z, Sun Y, Zhou X, Wu T, Tian Y, Wang Y. Synthesis and scale inhibitor performance of polyaspartic acid. J Environ Sci. 2011;23:S153–5. https://doi.org/10.1016/S1001-0742(11)61100-5.
Mackay EJ. Scale inhibitor application in injection wells to protect against damage to production wells: when does it work?. In: SPE European formation damage conference, May 25–27, Sheveningen, The Netherlands; 2005. https://doi.org/10.2118/95022-MS.
Malandrino A, Yuan MD, Sorbie KS, Jordan MM. Mechanistic study and modelling of precipitation scale inhibitor squeeze processes. In: SPE international symposium on oilfield chemistry, February 14–17, San Antonio, Texas; 1995. https://doi.org/10.2118/29001-MS.
Martinod A, Euvrard M, Foissy A, Neville A. Progressing the understanding of chemical inhibition of mineral scale by green inhibitors. Desalination. 2008;220:345–52. https://doi.org/10.1016/J.DESAL.2007.01.039.
Masler WF, Amjad Z. Itaconate ester copolymers as scale inhibitors. U.S. Patent No. 4,762,621. 9 Aug. 1988.
Menzri R, Ghizellaoui S, Tlili M. Calcium carbonate inhibition by green inhibitors: thiamine and pyridoxine. Desalination. 2017;404:147–54. https://doi.org/10.1016/J.DESAL.2016.11.005.
Migahed MA, Rashwan SM, Kamel MM, Habib RE. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants. J Mol Liq. 2016;224:849–58. https://doi.org/10.1016/J.MOLLIQ.2016.10.091.
Moghadasi J, Müller-Steinhagen H, Jamialahmadi M, Sharif A. Model study on the kinetics of oil field formation damage due to salt precipitation from injection. J Pet Sci Eng. 2004;43(3–4):201–17. https://doi.org/10.1016/J.PETROL.2004.02.014.
Moghadasi J, Sharif A, Müuller-Steinhagen H, Jamialahmadi M. Prediction of scale formation problems in oil reservoirs and production equipment due to injection of incompatible waters. Dev Chem Eng Miner Process. 2008;14(3–4):545–66. https://doi.org/10.1002/apj.5500140319.
Moudgil HK, Yadav S, Chaudhary RS, Kumar D. Synergistic effect of some antiscalants as corrosion inhibitor for industrial cooling water system. J Appl Electrochem. 2009;39(8):1339–47. https://doi.org/10.1007/s10800-009-9807-4.
Nasirtabrizi MH, Ziaei ZM, Jadid AP, Fatin LZ. Synthesis and chemical modification of maleic anhydride copolymers with phthalimide groups. Int J Ind Chem. 2013;4:11. https://doi.org/10.1186/2228-5547-4-11.
Okon A, Appah D, Akpabio J. Water coning prediction review and control: developing an integrated approach. J Sci Res Rep. 2017;14(4):1–24. https://doi.org/10.9734/JSRR/2017/33291.
Olajire AA. A review of oilfield scale management technology for oil and gas production. J Pet Sci Eng. 2015;135:723–37. https://doi.org/10.1016/J.PETROL.2015.09.011.
Popuri SR, Hall C, Wang CC, Chang CY. Development of green/biodegradable polymers for water scaling applications. Int Biodeterior Biodegrad. 2014;95:225–31. https://doi.org/10.1016/J.IBIOD.2014.04.018.
Przybylinski JL. Adsorption and desorption characteristics of mineral scale inhibitors as related to the design of squeeze treatments In: SPE international symposium on oilfield chemistry, February 8–10, Houston, Texas; 1989. https://doi.org/10.2118/18486-MS.
Quan Z, Chen Y, Wang X, Shi C, Liu Y, Ma C. Experimental study on scale inhibition performance of a green scale inhibitor polyaspartic acid. Sci China, Ser B: Chem. 2008;51(7):695–9. https://doi.org/10.1007/s11426-008-0063-y.
Rabaioli MR, Lockhart TP. Solubility and phase behavior of polyacrylate scale inhibitors. J Pet Sci Eng. 1996;15(2–5):115–26. https://doi.org/10.1016/0920-4105(96)00008-3.
Rahul R, Jha U, Sen G, Mishra S. Carboxymethyl inulin: a novel flocculant for wastewater treatment. Int J Biol Macromol. 2014;63:1–7. https://doi.org/10.1016/J.IJBIOMAC.2013.10.015.
Reddy MM, Hoch AR. Calcite crystal growth rate inhibition by polycarboxylic acids. J Colloid Interface Sci. 2001;235(2):365–70. https://doi.org/10.1006/JCIS.2000.7378.
Reis MIP, Gonçalves AD, da Silva FC, Jordão AK, Alves RJ, de Andrade SF, et al. Synthesis and evaluation of D-gluconamides as green mineral scales. Carbohydr Res. 2012;353:6–12. https://doi.org/10.1016/J.CARRES.2012.01.027.
Reno T, Endaryanto T. Modification of Gambier extracs as green inhibitor of calcium carbonate (CaCO3) scale formation. J. Water Process. Eng. 2017;18:1–6. https://doi.org/10.1016/J.JWPE.2017.05.004.
Richardson N, Holt B, Cook B. Cotelomer compounds. U.S. Patent No. 4,681,686. 21 Jul. 1987.
Roweton S, Huang SJ, Swift G. Poly(aspartic acid): synthesis, biodegradation, and current applications. J Environ Polym Degrad. 1997;5(3):175–81. https://doi.org/10.1007/bf02763661.
Sanders L, Hu X, Mavredaki E, Eroini V, Barker R, Neville A. Assessment of combined scale/corrosion inhibitors—a combined jar test/bubble cell. J Pet Sci Eng. 2014;118:126–39. https://doi.org/10.1016/J.PETROL.2014.04.008.
Scaled Solutions LTD. Squeeze Design [WWW Document]. http://www.scaledsolutions.com/Innovation R and D/Squeeze Design. Accessed 27 Apr 18.
Shaw SS, Sorbie KS. Synergistic properties of phosphonate and polymeric scale inhibitor blends for barium sulphate scale inhibition. In: SPE international oilfield scale conference and exhibition, May 14–15, Aberdeen, Scotland; 2014. https://doi.org/10.2118/169752-MS.
Shuler PJ. Mathematical model for the scale-inhibitor squeeze process based on the langmuir adsorption isotherm. In: SPE international symposium on oilfield chemistry, March 2–5, New Orleans, Louisiana; 1993. https://doi.org/10.2118/25162-MS.
Sorbie K, Mackay E. Mixing of injected, connate and aquifer brines in waterflooding and its relevance to oilfield scaling. J Pet Sci Eng. 2000;27:85–106. https://doi.org/10.1016/S0920-4105(00)00050-4.
Sorbie KS, Gdanski RD. A complete theory of scale inhibitor transport, adsorption/desorption and precipitation in squeeze treatments. In: SPE international symposium on oilfield scale, May 11–12, Aberdeen; 2005. https://doi.org/10.2118/95088-MS.
Sorbie KS, Laing N. How scale inhibitors work: mechanisms of selected barium sulphate scale inhibitors across a wide temperature range. In: SPE international symposium on oilfield scale, May 26–27, Aberdeen; 2004. https://doi.org/10.2118/87470-MS.
Sorbie KS, Yuan M, Jordan MM. Application of a scale inhibitor squeeze model to improve field squeeze treatment design. In: European petroleum conference, October 25–27, London; 1994. https://doi.org/10.2118/28885-MS.
Sousa MFB, Bertran CA. New methodology based on static light scattering measurements for evaluation of inhibitors for in bulk CaCO3 crystallization. J Colloid Interface Sci. 2014;420:57–64. https://doi.org/10.1016/J.JCIS.2014.01.001.
Sousa MFB, Signorelli F, Bertran CA. Fast evaluation of inhibitors for calcium carbonate scale based on pH continuous measurements in jar test at high salinity condition. J Pet Sci Eng. 2016;147:468–73. https://doi.org/10.1016/J.PETROL.2016.09.007.
Spicka K, Johnston CJ, Jordan MM, Nguyen L, Linares-Samaniego S, Sutherland L. The impact of organic acid on scale inhibitor/corrosion inhibitor interaction, a case study from West Africa. In: SPE International Symposium on Oilfield Chemistry, April 11–13, The Woodlands, Texas; 2011. https://doi.org/10.2118/141033-MS.
Sun Y, Xiang W, Wang Y. Study on polyepoxysuccinic acid reverse osmosis scale inhibitor. J Environ Sci. 2009;21:S73–5. https://doi.org/10.1016/S1001-0742(09)60041-3.
Sutherland L, Jordan M. Enhancing scale inhibitor squeeze retention with additives. In: SPE international oilfield scale conference and exhibition, May 11–12, Aberdeen, Scotland; 2016. https://doi.org/10.2118/179888-MS.
Thombre SM, Sarwade BD. Synthesis and biodegradability of polyaspartic acid: a critical review. J Macromol Sci Part A. 2005;42:1299–315. https://doi.org/10.1080/10601320500189604.
Tomson MB, Fu G, Watson MA, Kan AT. Mechanisms of mineral scale inhibition. SPE Prod Facil. 2003;18:192–9. https://doi.org/10.2118/84958-PA.
Tung NP, Phong NTP, Long BQK, Duy NH. Scale inhibitors for co-deposited calcium sulfate and calcium carbonate in squeeze process in white tiger oilfield. In: SPE international symposium on oilfield scale, May 26–27, Aberdeen; 2004 https://doi.org/10.2118/87467-MS.
Valiakhmetova A, Sorbie KS, Jordan MM, Boak LS. Novel studies on precipitated phosphate ester scale inhibitors for precipitation squeeze application. In: SPE international conference on oilfield chemistry, April 3–5, Montgomery, Texas; 2017. https://doi.org/10.2118/184511-MS.
Vazquez O, Corne D, Mackay E, Jordan MM. Automatic isotherm derivation from field data for oilfield scale-inhibitor squeeze treatments. SPE J. 2013;18:563–74. https://doi.org/10.2118/154954-PA.
Vazquez O, Fursov I, Mackay E. Automatic optimization of oilfield scale inhibitor squeeze treatment designs. J Pet Sci Eng. 2016a;147:302–7. https://doi.org/10.1016/J.PETROL.2016.06.025.
Vazquez O, Herrero P, Mackay E, Jordan M. Non-aqueous vs aqueous overflush scale inhibitor squeeze treatment in an oilfield offshore Norway. J Pet Sci Eng. 2016b;138:1–10. https://doi.org/10.1016/J.PETROL.2015.11.033.
Vazquez O, Mackay E, Sorbie K. A two-phase near-wellbore simulator to model non-aqueous scale inhibitor squeeze treatments. J Pet Sci Eng. 2012;82–83:90–9. https://doi.org/10.1016/J.PETROL.2011.12.030.
Vetter OJ. Oilfield scale-can we handle it? J Pet Technol. 1976;28:1402–8. https://doi.org/10.2118/5879-PA.
Vetter OJ. The chemical squeeze process some new information on some old misconceptions. J Pet Technol. 1973;25:339–53. https://doi.org/10.2118/3544-PA.
von Bonin W. Maleic acid anhydride copolymers and their preparation. U.S. Patent No. 4,390,672. 28 Jun. 1983.
Wang C, Shen T, Li S, Wang X. Investigation of influence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies. Desalination. 2014;348:89–93. https://doi.org/10.1016/J.DESAL.2014.06.017.
Wang Q, Liang F, AlNasser W, Al-Dawood F, Al-Shafai T, Al-Badairy H, et al. Laboratory study on efficiency of three calcium carbonate scale inhibitors in the presence of EOR chemicals. Petroleum. 2018. https://doi.org/10.1016/J.PETLM.2018.03.003.
Wang Y, Hou Y, Ruan G, Pan M, Liu T. Study on the polymerization of aspartic acid catalyzed by phosphoric acid. J Macromol Sci Part A. 2003;40:293–307. https://doi.org/10.1081/MA-120018116.
Wang Y, Li A, Yang H. Effects of substitution degree and molecular weight of carboxymethyl starch on its scale inhibition. Desalination. 2017;408:60–9. https://doi.org/10.1016/J.DESAL.2017.01.006.
Wildebrand C, Glade H, Will S, Essig M, Rieger J, Büchner KH, et al. Effects of process parameters and anti-scalants on scale formation in horizontal tube falling film evaporators. Desalination. 2007;204:448–63. https://doi.org/10.1016/J.DESAL.2006.03.547.
Yang L, Yang W, Xu B, Yin X, Chen Y, Liu Y, et al. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor. Desalination. 2017;416:166–74. https://doi.org/10.1016/J.DESAL.2017.05.010.
Yuan MD, Jamieson E, Hammonds P. Investigation of scaling and inhibition mechanisms and the influencing factors in static and dynamic inhibition tests. In: Corrosion 98, March 22–27, San Diego, California; 1998.
Zhang B, Zhang L, Li F, Hu W, Hannam PM. Testing the formation of Ca–phosphonate precipitates and evaluating the anionic polymers as Ca–phosphonate precipitates and CaCO3 scale inhibitor in simulated cooling water. Corros Sci. 2010;52:3883–90. https://doi.org/10.1016/J.CORSCI.2010.07.037.
Zhang H, Luo X, Lin X, Tang P, Lu X, Yang M, et al. Biodegradable carboxymethyl inulin as a scale inhibitor for calcite crystal growth: molecular level understanding. Desalination. 2016a;381:1–7. https://doi.org/10.1016/J.DESAL.2015.11.029.
Zhang P, Kan AT, Tomson MB. Oil field mineral scale control. In: Amjad Z, Demadis KD, editors. Mineral scales and deposits. Elsevier; 2015. p. 603–617. https://doi.org/10.1016/B978-0-444-63228-9.00024-3.
Zhang P, Shen D, Kan AT, Tomson MB. Phosphino-polycarboxylic acid modified inhibitor nanomaterial for oilfield scale control: transport and inhibitor return in formation media. RSC Adv. 2016b;6:59195–205. https://doi.org/10.1039/C6RA09973D.
Zhang P, Shen D, Ruan G, Kan AT, Tomson MB. Mechanistic understanding of calcium–phosphonate solid dissolution and scale inhibitor return behavior in oilfield reservoir: formation of middle phase. Phys Chem Chem Phys. 2016c;18:21458–68. https://doi.org/10.1039/C6CP03148J.
Zhang Y, Yin H, Zhang Q, Li Y, Yao P. Synthesis and characterization of novel polyaspartic acid/urea graft copolymer with acylamino group and its scale inhibition performance. Desalination. 2016d;395:92–8. https://doi.org/10.1016/J.DESAL.2016.05.020.
Zhao Y, Jia L, Liu K, Gao P, Ge H, Fu L. Inhibition of calcium sulfate scale by poly (citric acid). Desalination. 2016;392:1–7. https://doi.org/10.1016/J.DESAL.2016.04.010.
Acknowledgment
The authors are thankful to anyone who contributed to writing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no competing interest regarding publication of this manuscript. The preparation of the manuscript was done without receiving any external funding.
Additional information
Edited by Yan-Hua Sun
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mpelwa, M., Tang, SF. State of the art of synthetic threshold scale inhibitors for mineral scaling in the petroleum industry: a review. Pet. Sci. 16, 830–849 (2019). https://doi.org/10.1007/s12182-019-0299-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-019-0299-5