Abstract
Recently, nanoparticles have proven to enhance oil recovery on the core-flood scale in challenging high-pressure high-temperature reservoirs. Nanomaterials generally appear to improve oil production through wettability alteration and reduction in interfacial tension between oil and water phases. Besides, they are environmentally friendly and cost-effective enhanced oil recovery techniques. Studying the rheological properties of nanoparticles is critical for field applications. The instability of nanoparticle dispersion due to aggregation is considered as an unfavorable phenomenon in nanofluid flooding while conducting an EOR process. In this study, wettability behavior and rheological properties of surface-treated silica nanoparticles using internal olefins sulfonates (IOS20–24 and IOS19–23), anionic surfactants were investigated. Surface modification effect on the stability of the colloidal solution in porous media and oil recovery was inspected. The rheology of pure and surface-treated silica nanoparticles was investigated using a HPHT rheometer. Morphology and particle size distributions of pure and coated silica nanoparticles were studied using a field emission scanning electron microscope. A series of core-flood runs was conducted to evaluate the oil recovery factor. The coated silica nanoparticles were found to alter rheological properties and exhibited a shear-thinning behavior as the stability of the coated silica nanoparticles could be improved considerably. At low shear rates, the viscosity slightly increases, and the opposite happens at higher shear rates. Furthermore, the surface-modified silica nanoparticles were found to alter the wettability of the aqueous phase into strongly water-wet by changing the contact angle from 80° to 3° measured against glass slides representing sandstone rocks. Oil–water IFT results showed that the surface treatment by surfactant lowered the oil–water IFT by 30%. Also, the viscosity of brine increased from 0.001 to 0.008 Pa s by introducing SiO2 nanoparticles to the aqueous phase for better displacement efficiency during chemical-assisted EOR. The core-flood experiments revealed that the ultimate oil recovery is increased by approximately 13% with a surfactant-coated silica nanofluid flood after the conventional waterflooding that proves the potential of smart nanofluids for enhancing oil recovery. The experimental results imply that the use of surfactant-coated nanoparticles in tertiary oil recovery could facilitate the displacement efficiency, alter the wettability toward more water-wet and avoid viscous fingering for stable flood front and additional oil recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The decrease in the production rate in existing oil fields triggers concerns on enhancing the oil recovery factor. Most of the fields with residual oil saturation above thirty-percent are being abandoned (Zhang et al. 2008). Additional revenue can be achieved with a minor percent increase in the ultimate recovery factor. To attain the increasing oil recovery over the secondary baseline, the tertiary oil recovery technique has been designed (Kong and Ohadi 2010; Green and Willhite 1998). The residual oil after water flooding is indispensable and cannot be ignored particularly in the present situation of high energy demand. Further increase in oil recovery can be done after water flooding by utilizing various enhanced oil recovery (EOR) techniques (Maugeri 2009; Liebum et al. 2018). The decline in primary production of oil using conventional recovery approaches led to several developed techniques for EOR before the reservoir is abandoned. Recent studies of nanoparticles in EOR have offered hope to forthcoming EOR methods. Among them, silica nanoparticles are considered extensively (Ebrahim et al. 2019; Evdokimov et al. 2006; Hendraningrat et al. 2013; Roustaei et al. 2013). Various nanofluids could be prepared by dispersing them in different base-fluids (Sharma and Sangwai 2017; Sofla et al. 2019).
In view of declining production from oil fields and a time when recovering hydrocarbons are becoming more difficult, effective techniques are key to extract more oil from mature fields. In addition to the advent of nanotechnology, nanoparticles have emerged as promising materials to further improve various EOR techniques. Nanoparticles with at least a single dimension of 1–100 nm make them pass through porous media with relative ease. They are environmentally friendly especially in challenging temperature and pressure reservoirs (Lau et al. 2017; Olajire 2014; Singh and Mohanty 2014). When nanoparticles are introduced into the subsurface, they may create or break emulsions, or they may alter the porous media wettability (Romero-Zerón 2012; ShamsiJazeyi et al. 2014), thereby enhancing oil recovery. Surfactant flooding, on the other hand, has limitations as surfactants cannot be stabilized for long periods of time, especially in the presence of oil, high temperature and high salinity. The robustness of the surfactant can be increased significantly by the addition of surface-modified nanoparticles (Emrani et al. 2017; Singh and Mohanty 2014). Studying the rheological properties of nanoparticles is critical for field applications. An extensive distribution of nanoparticles indicates that the nanoparticles have enough specific surface area to transport around, which eventually causes a reduction in viscosity in case they are stable and not agglomerated (Ahmed et al. 2018a, b). Shear stress is a key indicator of categorizing Newtonian and non-Newtonian nanofluids (Ahmed et al. 2018a, b; Sharma and Sangwai 2017). Increasing the shear rate causes weaker particle–particle interactions to break hence causing nanofluids behaving like a Newtonian fluid (Hadadian et al. 2013). The viscosity of the nanofluid is not directly affected by particle aggregation. Nonetheless, with the small-scale accumulation of nanoparticles, the actual volume fraction becomes much lower than the effective volume fraction and as a result, nanofluids’ viscosity rises (Mishra et al. 2014; Rezaei et al. 2016). Apparently, the temperature has an absolute correlation with viscosity. Overall results revealed that with increasing temperature, a regular descending viscosity trend was observed. That suggests that viscosity reduces as the temperature rises, due to a reduction in inter-molecular forces among particles and the base-fluid (Thomas and Sobhan 2011).
Having considered the potential of silica nanoparticles in enhancing oil recovery, the performance evaluation of anionic surfactant-coated silica nanoparticles was evaluated. Although nanoparticles have widely been studied for EOR applications, however, a smart nanofluid like silica nanoparticles coated with anionic surfactant could be an emerging class of material that is superior to conventional nanoparticles. Anionic surfactant-coated silica nanoparticles may force injected fluid to move through the smaller pores by occupying high permeable zones and make the permeability uniform throughout the medium (Agi et al. 2018). It is known that the surfactant helps to improve the oil recovery by working at a microscopic level such that at the oil/water interface while nanoparticles being worked at a macroscopic level improves the overall displacement efficiency of the injected fluid (Emrani et al. 2017; Farid Ibrahim and Nasr-el-Din 2018). Hence, it is suggested that if both the surfactant and nanoparticles are used together, then the mobility ratio can relatively be much improved and less quantity of chemical (surfactant) will be required to accomplish the task of improving oil recovery from the depleted reservoirs because of the silica nanoparticles presence in the injected fluid.
Additionally, nanoparticles are assumed to reduce the effort of deformation required for an oil droplet to get displaced through the pore throat and help in mobilizing oil easily and effectively. Nanoparticles adsorb along with the interface of oil and water so as to reduce the IFT between oil/water and alter the wettability toward more water-wet (Romero-Zerón 2012; Souayeh et al. 2018). Moreover, the process of adsorption occurred between the nanoparticle and the surfactant on the rock surface and the surfactant double-layer formation together contributes to altering rock wettability (Souayeh et al. 2018). Conversely, the presence of nanoparticles in the aqueous phase also increases the injected fluid viscosity (Franco-Auguirre et al. 2018; Maestro et al. 2012). The increase in viscosity along with the IFT reduction and wettability alteration causes changes in the viscous and capillary forces; thus, increasing capillary numbers that ultimately could improve the oil recovery (Emadi et al. 2017; Manshad et al. 2017; Rezvani et al. 2018).
Although a lot of work has been done on the surface treatment of silica nanoparticles, no work related to olefin-based anionic surfactants for application in enhanced oil recovery has been reported yet according to the best of our knowledge. Hence, a detailed investigation is required to explore the newly developed olefin-based silica nanoparticles for EOR. The aim of this paper is to investigate the potential application of nanofluids in IOR/EOR processes. Silica nanoparticles were selected for the study, as they comprise more than 99% SiO2, which is the main constituent of sandstone rocks. Moreover, SiO2 is an environmentally friendly chemical. Additionally, these inorganic silica nanoparticles can be employed easily with regard to physical–chemistry properties (Miranda et al. 2012). In this work, the wettability and rheological properties of surface-treated silica nanoparticles using internal olefins sulfonates (IOS20–24 and IOS19–23), anionic surfactants have been investigated. For this, an HPHT rheometer was used to investigate the rheological properties before and after surfactant treatment. Finally, a core-flood experiment with the help of the Formation Evaluation System (FES-350) was conducted to confirm the potential of developed smart nanofluid in increasing ultimate oil recovery.
2 Materials and methods
Silica nanoparticles, trade name AEROSIL(R) OX 50, utilized in this study were provided by Evonik Malaysia Sdn Bhd. The nanoparticles had a typical silica-content more than 99% and an average size of 20–30 nm, specific surface area of 48 m2/g and 4.3 pH value. Two commercially used internal olefin sulfonates (IOS), i.e., ENORDET™ O-242 (IOS20–24)—Surf X and ENORDETTM O-342 (IOS19–23)—Surf Y, were provided by Shell Chemicals. Surf X contains 20 to 24 carbon atoms with a sulfonate group attached to their head, while Surf Y consists of 19 to 23 carbon atoms and a sulfonate group attached to it. The densities of these two IOS surfactants Surf X and Surf Y are 0.9986 and 0.9987 g/cm3, respectively. The only difference found between them is the length of the carbon chain. Both surfactants are applicable for low/medium salinity and are thermally stable up to reservoir conditions of 200 °C. On their own, IOS has limited aqueous solubility, particularly in brines containing higher levels of Ca2+ and Mg2+ ions (‘hard’ brines).
A degassed Malaysian crude oil used in this study had an API density of approximately 40.2° at standard temperature and a viscosity of 3.53 cP. The crude oil used was free from any type of additives and was filtered prior to being used for experiments so as to ensure the consistency of the crude oil components. Sodium chloride (3.5% NaCl, supplied by Sigma-Aldrich) was used to prepare brine of salinity of 35,000 ppm in accordance with the salinity of seawater used in Malaysian reservoirs.
2.1 Surface activation of silica nanoparticles
For the surface modification, 20 g of pure silica nanoparticles was mixed with 200 mL of 2 wt% prepared surfactant solutions for approximately 48 h at 50 °C. The modified particles were separated from the mixture using a Heraeus MULTIFUGE X1R Centrifuge at approximately 8000 rpm for 20 min. Later, the prepared samples were washed with distilled water and filtered repeatedly so that the untreated silica nanoparticles were washed out. Lastly, the treated silica nanoparticles were placed in a laboratory oven at 50 °C for 72 h to get dried. The process flow is demonstrated in Fig. 1 (van der Merwe et al. 2011).
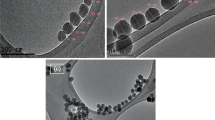
The size of silica nanoparticles derived from SEM images was 40 ± 6.2 nm (nanoparticle number = 100), and their associated size distributions are shown in Fig. 2. The particles were dispersed in ethanol and ultra-sonicated to remove agglomeration. After TEM analysis, the particle diameter for 100 particles was calculated. The size distribution shows that the average size was around 40 nm. The wettability of treated and untreated particles is controlled with the help of an extensive balance of hydrophobic and electrostatic interactions among the particle surface and the surfactant. These interactions play an important role in the structure of the surfactant–particle nanocomposite interfacial layer (Maestro et al. 2012).
2.2 Effect of surface treatment on the morphology of silica nanoparticles
Figures 3, 4 and 5 illustrate the field emission scanning electron microscopy (FESEM) images of silica nanoparticles before and after IOS treatments. These images suggest that the silica nanoparticles were coated with surfactants. The presence of the surfactant layers absorbed on silica nanoparticles would reduce the attractive forces between the particles and provided significant dispersion stability in the aqueous phase as the particles were found to be agglomerated before the modification. It was also noted that silica particles were of nano-size (averaging 90 nm). This ultra-small size can overcome the challenge of pore plugging in porous media as particles can easily pass through the pore throats without severe permeability reduction. This ability can allow nanoparticles to have more contact with swept zones thus increasing the macroscopic sweep efficiency and then the effectiveness of injected fluids for EOR.
Figure 3 shows the results of FESEM with 30 K magnification for untreated silica nanoparticles in which agglomeration of particles was observed due to strong attractive forces among them. Under the same magnification, when the surface of silica nanoparticles was modified (see Figs. 4 and 5), the attraction forces between the particles were found to be reduced as the particles were seemed to be relatively more dispersed after treatment.
Characterization suggested that after treatment the nanoparticles possess high surface energies because of high ratio of surface to volume along with van der Waals attraction or magnetic attractions, while there is insufficient repulsion (e.g., electrostatic or configurational entropic) between the particles that leads them to form a secondary shape that is relatively large than their primary size (Nandanwar et al. 2011).
2.3 Wettability analysis
Contact angles were directly measured on glass slices representing sandstone rock using IFT-700 (IFT pendant drop) equipment at room conditions before and after surface treatment. The contact angle is one of the most commonly considered methods to estimate the wetting characteristic of rocks and is defined as “the angle formed at the boundary surface by a liquid with solid and is measured through the liquid to solid” (Singh and Mohanty 2016). The contact angle expresses the tendency of a liquid to get adhered to the solid surface in the presence of another immiscible liquid. Low contact angle shows stronger wetting characteristic, i.e., 0° is completely wetting and 180° completely non-wetting. The system of measurement consists of rock/glass surface, oil and nanofluids/brine.
2.4 Rheological property measurements
In EOR applications, mobility ratio is one of the most important parameters and can be defined as the ratio between the mobility of the displacing fluid to the mobility of the displaced fluid (Liebum et al. 2018). In flooding cases, the displacing fluid is then injected (nanofluid) and the displaced one is the residual oil present inside the core. The shear rate always affects the viscosity whenever the fluid is injected into the porous media. Therefore, the displacing phase viscosity is a very important factor in deploying nanofluid in porous media. Therefore, rheological properties were evaluated with an HPHT Modular Compact Rheometer (MCR 302). Measurements were taken at ambient temperature with the help of DG 26.7 double-gap geometry. Before taking measurements, samples were set to equilibrate at the desired temperature for approximately 10 min.
2.5 Core-flood experimental setup
A Formation Evaluation System (FES 350) supported by Vinci Technologies was used to conduct core-flood experiments. FES 350 has the capability to inject multiple fluids with maximum pore pressure up to 5000 psi, confining pressure up to 10,000 psi and temperature up to 200 °C. Figure 6 shows a schematic diagram of the core-flood setup.
2.6 Core preparation
For all the core-flood experiments, two buff Berea sandstone cores (3″ length and 1.5″ diameter) were used. These experiments were aimed to reveal the capability of surface-modified nanofluids for EOR applications. Different core properties including porosity and permeability were measured in the core laboratory using Poroperm COVAL 30. After the measurements of core properties, each core was first placed in a beaker containing distilled water and the air was evacuated from the chamber using a vacuum desiccator that allows water to enter the core easily.
Table 1 summarizes the physical parameters of cores obtained from Poroperm COVAL 30.
The experimental procedure used for core flooding is as follows:
- (1)
The water-saturated core was mounted in the core holder and then flooded with brine to ensure full-core brine saturation at given temperature–pressure conditions. The injection rate and the confining pressures were 0.2 mL/min and around 3000 psi, respectively.
- (2)
The brine-saturated core was flooded with crude oil to establish initial water saturation (Swi) at a flow rate of 0.2 mL/min until no more brine was produced. In order to reach that situation, nearly 2 PV (pore volume) was required.
- (3)
Brine (approximately 2 PV) was then injected into the oil-saturated core at a flow rate of 0.2 mL/min until no more oil was produced. The residual oil saturation after water production was termed Sor.
- (4)
After water flooding, nanofluid injection (2 PV) at a rate of 0.2 mL/min was started as a tertiary recovery technique. The nanofluid concentration was kept 0.1 wt% on the basis of earlier screening. The decreased residual oil saturation along with the ultimate oil recovery was evaluated for two cases: pure silica nanofluid flooding and flooding with surfactant-coated silica nanofluid.
- (5)
The last step followed was a 2 PV post-waterflood at a flow rate of 0.2 mL/min.
3 Results and discussion
3.1 Contact angles
For measuring contact angle, 0.05 wt% and 0.10 wt% of nanofluids were chosen. The sessile and pendant drop method (IFT-700 tensiometer, France) was used to measure the contact angle of pure and surface-modified silica nanoparticles in the liquid/liquid system. The contact angle was measured on nanofluids-treated glass slides representing sandstone samples. The glass was first cleaned with methanol followed by distilled water. Each oil drop was made at the different locations of the glass slide at similar conditions.
The contact angle of the initial oil drop over the glass slice was observed to be 80° (average of left and right angles). Having dispersed the glass piece separately at room temperature into different concentration nanofluids with the aging time of approximately 48 h followed by drying in a heated oven for 24 h, the reduction in contact angle was found in the presence of silica nanofluids (see Fig. 7). On repeating the measurements several times, similar results were observed with the tolerance of ± 3 degrees of contact angle.
In addition, the contact angles of surfactant-coated silica nanofluids (Surf Y- and Surf X-coated silica nanofluids) with oil drop were observed to decline in comparison with when it was initially measured with brine, as shown in Fig. 8, which represents the contact angle measurements of crude oil and brine/nanofluids at various concentrations on the silica-glass surface. It can be seen that the contact angle was observed to be reduced significantly from 80° to the least values of 3°–2° when the glass surface was soaked with the surfactant-coated silica nanofluids. The trend suggested that both surface-coated silica nanofluids altered the rock wettability toward strongly water-wet, evidenced by a reduction in contact angle when the silica nanofluid was introduced (Ahmed et al. 2018a, b; Hendraningrat et al. 2013; Morrow 1976). On the surface of solids very close to the drop’s three-phase (solid/liquid/nano) contact line, nanoparticles advance along the surface as monolayer particles by creating ordered structures. If the angle of contact with the aqueous phase is zero, it implies that the nanofluid possesses notably huge surface area that helps them to totally spread throughout the surface and the fluid contains high surface energies with adsorption that leads to wettability alteration of the system (Manshad et al. 2017; Nwidee et al. 2017; Souayeh et al. 2018). It was also observed that as the nanoparticle concentration increases, the water wetness of the rock also increases which is due to the high electrostatic repulsion among particles that causes nanofluid to spread along the rock surface as a result the contact angle reduces. Besides this, with an increase in the silica nanoparticle concentration in the aqueous system, strongly water-wet behavior of solids can be seen (Maestra et al. 2012; Rao 2010; Souayeh et al. 2018).
3.2 Interfacial tensions
Goniometer RAME-HART 260-F4 was used to measure the interfacial tension (IFT) between degassed crude oil as the droplet phase and brine/nanofluids as the external phase at 25, 50 and 85 °C (see Fig. 9). The newly formed oil/water interface was exposed to the aqueous phase containing nanoparticles for 24 h before measurements of IFT. In the experimental process, an oil droplet was generated from the end of a capillary needle in a nanofluid at experimental pressure and temperature. The IFT value was calculated by analyzing the complete shape of the oil droplet by an accurate video system and analysis software.
Figures 10, 11 and 12 show the IFT between nanofluids/crude oil at different temperatures before and after surface treatments. The IFT decreased from 17.10 mN/m (reference value) at 25 °C to a minimum of approximately 6 mN/m as the nanoparticles were introduced into the brine at 25 °C. The reason for a reduction in IFT may be due to the settling of nanoparticles at the oil and water interface (Farid Ibrahim and Nasr-el-Din 2018). However, for 0.05 wt% surfactant-treated nanofluids, the IFT decreased to 10.12 mN/m and 9 mN/m at 25 °C, respectively, in the case of Surf X- and Surf Y-coated nanoparticles. Additionally, it was noticed that the IFT reduced with an increase in the concentration of nanoparticles.
Moreover, as the temperature increased to 85 °C, the IFT decreased significantly to 7.16 and 5.13 mN/m for 0.03 wt% Surf X- and Surf Y-coated silica nanofluids (Fig. 12). Declining IFT results in a reduction in capillary pressure that reveals better efficiency and oil recovery (Andreassen 2015). This causes the remaining oil saturation to be decreased, and the oil trapped by capillary pressure will be released due to a reduction in IFT (Ahmed et al. 2017a, b, 2018a, b).
Therefore, it can be reported that Surf Y-treated nanoparticles are proved to be more effective than the other untreated. Moreover, the Surf Y-treated silica nanofluid had the least IFT value of 5.13 mN/m as the temperature increased to 85 °C.
The results suggested that using 0.10 wt% surfactant-treated nanofluids the relatively stable IFTs, the lowest values are found between the temperature 25 and 85 °C that also consolidates the earlier research work done on EOR using nanoparticles. It has also been noticed that the interfacial tension should be as lowered as possible to make the injected fluid capable of enhancing residual oil recovery from porous media (Andreassen 2015; Ahmed et al. 2017a, b, 2018a, b).
3.3 Rheological properties
Fluid rheology is a very critical parameter to be understood. The rheological properties of nanofluids were measured with an HPHT Modular Compact Rheometer (MCR 302) at ambient temperature with the help of DG 26.7 double-gap geometry. Before measurements, samples were set to equilibrate the temperature for approximately 10 min.
3.3.1 Variation in nanofluid viscosity with shear rate
The viscosities of 0.10 wt% (1000 ppm) nanofluids (pure silica nanoparticle solution, Surf X- and Surf Y-coated nanoparticle solutions) were measured at different shear rates (0–100 s−1), and the experimental results are shown in Fig. 13. It can be seen that for the same concentration, the Surf X-coated nanofluid has the highest viscosity followed by the Surf Y-coated nanofluid and then the pure silica nanofluid (valid under the shear rate of 30 s−1). A huge distinction is noticed in the viscosity values at low shear rates (< 30 s−1). At high shear rates (> 30 s−1), the nanofluid viscosity values are found to be lying close to each other. This behavior is due to the breaking of the aggregation network occurring when the shear forces overpass aggregation forces (Jamshidi et al. 2012; Rao 2010).
The viscosity of the Surf Y-coated silica nanofluid shows a plateau (> 30 s−1) after an exponential decrease (< 30 s−1). This behavior of the Surf Y-coated silica nanofluid makes it suitable for EOR applications to have stable displacement front. On the other hand, the viscosity of the Surf X-coated silica nanofluid increases after the shear rate higher than 85 s−1 that may be due to aggregation of particles at high shear rates. This makes Surf X-coated silica nanofluid unsuitable for EOR applications. However, most of the nanofluids display shear-thinning that can also be seen in the surfactant-coated silica nanofluids. A reduction in viscosity is assumed to happen when the fluid is subjected to applied shearing in addition to higher applied stress, leading to a Bingham fluid that results in lowered viscosity (Ahmed et al. 2018a, b; Jamshidi et al. 2012). Shear-thinning makes the injected fluid non-Newtonian as the viscosity decreases with increasing shear strain, which makes the injected fluid more easily flow into water channeling or thief zones thus enhancing the oil recovery (Ahmed et al. 2017a, b, 2018a, b).
3.3.2 Variation in nanofluid viscosity with temperature
The effect of temperature on the viscosity of three nanofluids, pure, Surf X- and Surf Y-coated silica nanofluids was investigated. The experimental results shown in Fig. 14 reveal that the nanofluid viscosity reduces with an increase in temperature. The reason for this phenomenon is that increasing temperature may cause a decrease in the inter-particle/inter-molecular forces (Ahmed et al. 2016, 2018a, b; Jamshidi et al. 2012). It can be seen that above a transition temperature around 64 °C, the viscosity of both surfactant-coated silica nanofluids (Surf X and Surf Y) increases sharply. This suggests that the nanoparticles formed a gel-type structure above 64 °C that might force the injected fluid to pass through the smaller pores that had been left un-swept in the case of displacement by the pure silica particle fluid. Moreover, initially at a constant shear rate of 10 s−1, the repulsive interaction among particles allows them to disperse in the fluid until the temperature reaches 64 °C. Furthermore, at high temperatures, the attractive interactions dominate thereby particles seem to form a gel-type structure and aggregate through hydrogen bonding and polymer entanglement, which are progressively disrupted under the influence of applied shear stress (Ahmed et al. 2016; Nguyen et al. 2007; Wang and Dong 2009).
3.4 Effect of surface-modified nanofluid on oil recovery
The two-phase displacement experiments were carried out on Berea core plugs with the help of the FES-350 core-flood system. The core objective of these laboratory tests was to analyze the potential of surface-modified silica nanoparticle solutions (smart nanofluids) for enhancing oil recovery at the given reservoir conditions (119 °C and 2200 psi).
Figure 15 illustrates the differential pressure changes during the core-flooding runs of pure and surface-modified silica nanofluid floods. A slight increase in differential pressure across the core was observed at the breakthrough of 2 PV of 0.10 wt% nanofluids in both pure and surfactant-coated silica nanofluid floods. This increment could be a result of the increase in viscosity of nanofluids (Hou et al. 2015) and the slight reduction in permeability due to nanoparticle entrapment in relatively bigger pore throats of water-channels hence diverting the injected fluid flow toward un-swept pore throats (Ahmed et al. 2017a, b).
The adsorption of nanoparticles caused an alteration of rock and fluid properties, i.e., wettability and IFT (Hou et al. 2015; Myers 2005). Consequently, the ultimate oil recovery also significantly increased by nanofluid injection. The experimental results (Table 2) show that after brine injection into Berea cores I and II, the primary oil recoveries were found to be 32.0% and 39.1%, respectively. The increases in the ultimate oil recoveries were 9.1% and 21.7%, respectively, for pure and surface-modified silica nanofluid floods.
Initially, the core is in water-wet condition, i.e., the surface of the rock is covered with water film and the existence of oil is in the globules. However, after the surfactant-coated silica nanofluid is injected into the core, the water film may become unstable because more silica nanoparticles along with surfactants coated on the particles tend to be adsorbed at the interface of rock and water compared to that at the interface of oil and water (Farid Ibrahim and Nasr-el-Din 2018; Ahmed et al. 2017a, b; Hou et al. 2015; Myers 2005). Consequently, this liquid film (water film and the existence of oil is in the globules) instability could lead to the formation of a mixed-wet state that is favorable for displacing oil through porous media (Farid Ibrahim and Nasr-el-Din 2018; Ahmed et al. 2017a, b; Salathiel 1973). An additional recovery of approximately 13% over the pure silica nanoparticles was achieved after the injection of Surf Y-coated silica nanofluid (see Table 2). This proves the potential of surface-modified nanofluids for enhancing oil recovery. The phenomena of a slight pressure build-up due to the injection of surface-modified silica nanofluid could be one of the main reasons for the increment of oil recovery. This phenomenon is named as log-jamming, which is a kind of temporary accumulation of nanoparticles and is beneficial to improving the performance of nanofluid flooding (Franco-Aguirre et al. 2018; Skauge et al. 2010). This mechanism results in a pressure build-up in the pores, forcing the trapped oil out from the nearby pore throats. As the oil frees, the pressure around the surroundings drops back, and the plugging disappears gradually that causes the injected nanofluid to start flowing with water again. Furthermore, altering wettability and reducing IFT could be the major contributing factors in improving oil recovery but not limited to them only.
4 Conclusions
-
(1)
In this paper, the rheological properties and IFT reduction in pure and surfactant-coated silica nanofluids were investigated. Two different internal olefin sulfonates (IOS20–24 and IOS19–23) were used to modify silica nanoparticles. The following main conclusions can be drawn on the basis of the experimental results: The surface-modified silica nanoparticles altered the rock wettability from weakly water-wet toward more water-wet, which was evidenced by the reduction in the contact angle from 80° to the least values of 3°–2°.
-
(2)
The addition of 0.30 wt% surface-modified silica nanofluid lowered the oil/water IFT as the temperature rises, which is beneficial to EOR.
-
(3)
The surface-modified silica nanoparticles could alter the rheological properties of fluids due to as the stability of the coated silica nanoparticles that has been observed by their shear-thinning behavior. This behavior makes the injected fluid non-Newtonian as the nanofluid viscosity decreases with increasing shear strain. This is conducive to the injected fluid flowing into water channeling or thief zones and then the enhanced oil recovery.
-
(4)
Both the pure and surface-modified silica nanofluids could enhance the ultimate oil recovery from sandstone cores by about 9.1% and 21.7% of the original oil in place, respectively.
References
Agi A, Junin R, Gbadamosi A. Mechanism governing nanoparticle flow behavior in porous media: insight for enhanced oil recovery applications. Int Nano Lett. 2018;8(2):49–77. https://doi.org/10.1007/s40089-018-0237-3.
Ahmed AA, Saaid IM, Akhir NAM. Effect of surfactants and grafted copolymer on stability of bentonite particles dispersion in brine system. ARPN J Eng Appl Sci. 2016;11(7):4822–7.
Ahmed AA, Saaid IM, Akhir NAM. Graft copolymerization of N-isopropylacrylamide and acrylic acid on bentonite colloids for in-depth fluid diversion. Energy Fuels. 2017a;31:3537–45. https://doi.org/10.1021/acs.energyfuels.6b02507.
Ahmed A, Saaid IM, Tunio AH, Pilus RM, Mumtaz M, Ahmad I. Investigation of dispersion stability and IFT reduction using surface-modified nanoparticle: enhanced oil recovery. J Appl Environ Biol Sci. 2017b;7:56–62.
Ahmed A, Saaid IM, Pilus RM, Ahmed AA. Rheology and interfacial tension of internal olefin sulphonate coated nano-silica for enhanced oil recovery. Int J Adv Sci Eng Technol. 2018a;6(1):96–101.
Ahmed A, Saaid IM, Pilus RM, Ahmed AA, Tunio AH, Baig MK. Development of surface-treated nanosilica for wettability alteration and interfacial tension reduction. J Dispers Sci Technol. 2018b. https://doi.org/10.1080/01932691.2017.1417133.
Andreassen L. Master thesis: Nanoparticle effect on Interfacial Properties related to Enhanced Oil Recovery, 2015. https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/2350975.
Ebrahim T, Mohsen VS, Mahdi SM, et al. Performance of low-salinity water flooding for enhanced oil recovery improved by SiO2 nanoparticles. Pet Sci. 2019;16(2):357–65. https://doi.org/10.1007/s12182-018-0295-1.
Emadi S, Shadizadeh SR, Manshad AK, Rahimi AM, Mohammadi AH. Effect of nano silica particles on interfacial tension (IFT) and mobility control of natural surfactant (Cedr extraction) solution in enhanced oil recovery process by nano-surfactant flooding. J Mol Liq. 2017;248:163–7. https://doi.org/10.1016/j.molliq.2017.10.031.
Emrani AS, Ibrahim AF, Nasr-El-Din HA. Mobility control using nanoparticle-stabilized CO2 foam as a hydraulic fracturing fluid. In: SPE Europec featured at 79th EAGE conference and exhibition. Society of Petroleum Engineers; 2017. https://doi.org/10.2118/185863-MS.
Evdokimov IN, Eliseev NY, Losev AP, Novikov MA. Emerging petroleum-oriented nanotechnologies for reservoir engineering. In: SPE Russian oil and gas technical conference and exhibition. 2006. https://doi.org/10.2118/102060-MS.
Farid Ibrahim A, Nasr-El-Din H. Stability improvement of CO2 foam for enhanced oil recovery applications using nanoparticles and viscoelastic surfactants. In: SPE Trinidad and Tobago section energy resources conference. Society of Petroleum Engineers; 2018. https://doi.org/10.2118/191251-MS.
Franco-Aguirre M, Zabala RD, Lopera SH, Franco CA. Cortés FBInteraction of anionic surfactant-nanoparticles for gas-wettability alteration of sandstone in tight gas-condensate reservoirs. J Nat Gas Sci Eng. 2018;51:53–64. https://doi.org/10.1016/j.jngse.2017.12.027.
Green DW, Willhite GP. Enhanced oil recovery, vol. 6. Richardson: Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers; 1998.
Hadadian M, Samiee S, Ahmadzadeh H, Goharshadi EK. Nanofluids for heat transfer enhancement—a review. Phys Chem Res. 2013;1:1–33. https://doi.org/10.22036/PCR.2013.2791.
Hendraningrat L, Li S, Torsæter O. A coreflood investigation of nanofluid enhanced oil recovery. J Pet Sci Eng. 2013;111:128–38. https://doi.org/10.1016/j.petrol.2013.07.003.
Hou BF, Wang Y, Huang Y. Mechanistic study of wettability alteration of oil-wet sandstone surface using different surfactants. Appl Surf Sci. 2015;330:56–64. https://doi.org/10.1016/j.apsusc.2014.12.185.
Jamshidi N, Farhadi M, Ganji D, Sedighi K. Experimental investigation on viscosity of nano fluids. Int J Eng. 2012;25:201–9.
Kong X, Ohadi M. Applications of micro and nano technologies in the oil and gas industry-overview of the recent progress. In: Abu Dhabi international petroleum exhibition and conference. 2010. https://doi.org/10.2118/138241-MS.
Lau HC, Yu M, Nguyen QP. Nanotechnology for oilfield applications: challenges and impact. J Pet Sci Eng. 2017;157:1160–9. https://doi.org/10.1016/j.petrol.2017.07.062.
Liebum MM, Hirasaki G, Nguyen QP. A systematic rheological study of alkyl amine surfactants for fluid mobility control in hydrocarbon reservoirs. Pet Sci. 2018;15(3):538–51. https://doi.org/10.1007/s12182-018-0217-2.
Maestro A, Guzmán E, Santini E, et al. Wettability of silica nanoparticle–surfactant nanocomposite interfacial layers. Soft Matter. 2012;8(3):837–43. https://doi.org/10.1039/C1SM06421E.
Manshad AK, Rezaei M, Moradi S, Nowrouzi I, Mohammadi AH. Wettability alteration and interfacial tension (IFT) reduction in enhanced oil recovery (EOR) process by ionic liquid flooding. J Mol Liq. 2017;2017(248):153–62. https://doi.org/10.1016/j.molliq.2017.10.009.
Maugeri L. Squeezing more oil from the ground. Sci Am. 2009;301:56–63. https://doi.org/10.1038/scientificamerican1009-56.
Miranda CR, Lara LS de, Tonetto BC. Stability and mobility of functionalized silica nanoparticles for enhanced oil recovery applications. In: SPE international oilfield nanotechnology conference and exhibition. 2012. https://doi.org/10.2118/157033-MS.
Mishra PC, Mukherjee S, Nayak SK, Panda A. A brief review on viscosity of nanofluids. Int Nano Lett. 2014;4:109–20. https://doi.org/10.1007/s40089-014-0126-3.
Morrow NR. Capillary pressure correlations for uniformly wetted porous media. J Can Pet Technol. 1976. https://doi.org/10.2118/76-04-05.
Myers D. Surfactant science and technology. Hoboken: Wiley; 2005.
Nandanwar S, Chakraborty M, Mukhopadhyay S, Shenoy K. Stability of ruthenium nanoparticles synthesized by solvothermal method. Cryst Res Technol. 2011;46:393–9. https://doi.org/10.1002/crat.201100025.
Nguyen C, Desgranges F, Roy G, Galanis N, Maré T, Boucher S. Temperature and particle-size dependent viscosity data for water-based nanofluids–hysteresis phenomenon. Int J Heat Fluid Flow. 2007;28:1492–506. https://doi.org/10.1016/j.ijheatfluidflow.2007.02.004.
Nwidee LN, Lebedev M, Barifcani A, Sarmadivaleh M, Iglauer S. Wettability alteration of oil-wet limestone using surfactant–nanoparticle formulation. J Colloid Interface Sci. 2017;504:334–45. https://doi.org/10.1016/j.jcis.2017.04.078.
Olajire AA. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: prospects and challenges. Energy. 2014;77:963–82. https://doi.org/10.1016/j.energy.2014.09.005.
Rao Y. Nanofluids: stability, phase diagram, rheology, and applications. Particuology. 2010;8:549–55. https://doi.org/10.1016/j.partic.2010.08.004.
Rezaei A, Abdi-Khangah M, Mohebbi A, Tatar A, Mohammadi AH. Using surface modified clay nanoparticles to improve rheological behavior of hydrolized polyacrylamide (HPAM) solution for enhanced oil recovery with polymer flooding. J Mol Liq. 2016;222:1148–56. https://doi.org/10.1016/j.molliq.2016.08.004.
Rezvani H, Khalilnezhad A, Ganji P, Kazemzadeh Y. How ZrO2 nanoparticles improve the oil recovery by affecting the interfacial phenomena in the reservoir conditions? J Mol Liq. 2018;252:158–68. https://doi.org/10.1016/j.molliq.2017.12.138.
Romero-Zerón L. Introduction to enhanced oil recovery (EOR) processes and bioremediation of oil-contaminated sites. Croatia: InTech; 2012.
Roustaei A, Saffarzadeh S, Mohammadi M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt J Pet. 2013;22:427–33. https://doi.org/10.1016/j.ejpe.2013.06.010.
Salathiel R. Oil recovery by surface film drainage in mixed-wettability rocks. J Petrol Technol. 1973;25:1216–24. https://doi.org/10.2118/4104-PA.
ShamsiJazeyi H, Miller CA, Wong MS, Tour JM, Verduzco R. Polymer-coated nanoparticles for enhanced oil recovery. J Appl Polym Sci. 2014;131:2. https://doi.org/10.1002/app.40576.
Sharma T, Sangwai JS. Silica nanofluids in polyacrylamide with and without surfactant: Viscosity, surface tension, and interfacial tension with liquid paraffin. J Pet Sci Eng. 2017;152:575–85. https://doi.org/10.1016/j.petrol.2017.01.039.
Singh R, Mohanty KK. Synergistic stabilization of foams by a mixture of nanoparticles and surfactants. In: SPE improved oil recovery symposium. Society of Petroleum Engineers; 2014. https://doi.org/10.2118/169126-MS.
Singh R, Mohanty KK. Foams with wettability-altering capabilities for oil-wet carbonates: a synergistic approach. SPE J. 2016;21:1126–39. https://doi.org/10.2118/175027-PA.
Skauge T, Spildo K, Skauge A. Nano-sized particles for EOR. In: SPE improved oil recovery symposium. Tulsa, OK, USA, 24–28 April 2010. https://doi.org/10.2118/129933-MS.
Sofla SJD, James LA, Zhang Y. Understanding the behavior of H + -protected silica nanoparticles at the oil-water interface for enhanced oil recovery (EOR) applications. J Mol Liq. 2019;274:98–144. https://doi.org/10.1016/j.molliq.2018.09.049.
Souayeh M, Al-Maamari RS, Aoudia M, Karimi M, Hadji M. Experimental investigation of wettability alteration of oil-wet carbonates by a non-ionic surfactant. Energy Fuels. 2018;32(11):11222–33. https://doi.org/10.1021/acs.energyfuels.8b02373.
Thomas S, Sobhan CBP. A review of experimental investigations on thermal phenomena in nanofluids. Nanoscale Res Lett. 2011;6(1):377. https://doi.org/10.1186/1556-276X-6-377.
van der Merwe E, Prinsloo LC, Kruger RA, Mathebula LC. Characterization of coal fly ash modified by sodium lauryl sulphate. In: World of Coal Ash Conference Proceedings. Denver, Colorado; 2011. p. 16.
Wang J, Dong M. Optimum effective viscosity of polymer solution for improving heavy oil recovery. J Pet Sci Eng. 2009;67:155–8. https://doi.org/10.1016/j.petrol.2009.05.007.
Zhang L, Yuan J, Liang H, Li K. Energy from abandoned oil and gas reservoirs. In: SPE Asia pacific oil and gas conference and exhibition. 2008. https://doi.org/10.2118/115055-MS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Yan-Hua Sun
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahmed, A., Saaid, I.M., Ahmed, A.A. et al. Evaluating the potential of surface-modified silica nanoparticles using internal olefin sulfonate for enhanced oil recovery. Pet. Sci. 17, 722–733 (2020). https://doi.org/10.1007/s12182-019-00404-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-019-00404-1