Abstract
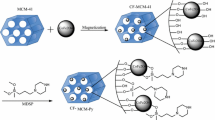
An effective sorbent of 1-(2-Pyridylazo)-2-naphthol-functionalized mesoporous silica has been prepared to simultaneous separation and preconcentration of lead and cadmium ions in aqueous solution. Structural characterization of 1-(2-Pyridylazo)-2-naphthol-functionalized organic–inorganic hybrid mesoporous materials was conducted by Fourier transform infrared spectroscopy, transmission electron microscopy, N2 adsorption–desorption measurement, X-ray diffraction, and elemental and thermal analysis, which confirmed the successful grafting of organic moiety on mesoporous silica. The affecting parameters on adsorption and desorption steps were optimized by Box-Behnken design through response surface methodology. Three variables (pH value, sorption time, and amount of the sorbent) were selected as the main factors affecting sorption step, while four variables (type of eluent, eluent volume, eluent concentration, and elution time) were selected for desorption step in the optimization study. The optimized values by this optimization method were 10 mg, 8 min, 6.3, HCl, 1.6 mL, 1.2 mol L−l HCl, and 10 min, for amount of sorbent, sorption time, pH of solution, type, volume, and concentration of the eluent, and elution time, respectively. Under the optimized conditions, the detection limits of the proposed method for lead and cadmium ions were found to be 0.9 and 0.04 μg L−1, respectively, while the relative standard deviation (RSD) for five replicate measurements was calculated to be <3 % for both ions. For proving that the proposed method is reliable, a wide range of food, soil, and water samples with different and complex matrixes was used.







Similar content being viewed by others
References
Asadollahzadeh M, Tavakoli H, Torab-Mostaedi M, Hosseini G, Hemmati A (2014) Response surface methodology based on central composite design as a chemometric tool for optimization of dispersive-solidification liquid–liquid microextraction for speciation of inorganic arsenic in environmental water. Talanta 123:25–31
Baghdadi M, Shemirani F (2008) Cold-induced aggregation microextraction: a novel sample preparation technique based on ionic liquids. Anal Chim Acta 613:56–63
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Taghizade M, Baghayi L, Salarian M (2012a) Simultaneous separation and determination of trace amounts of Cd (II) and Cu (II) in environmental samples using novel diphenylcarbazide modified nanoporous silica. Talanta 89:455–461
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Tootoonchi A, Dahaghin Z (2012b) Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles. Microchim Acta 178:261–268
Bagheri A, Taghizadeh M, Behbahani M, Asgharinezhad AA, Salarian M, Dehghani A, Ebrahimzadeh H, Amini MM (2012c) Synthesis and characterization of magnetic metal-organic framework (MOF) as a novel sorbent, and its optimization by experimental design methodology for determination of palladium in environmental samples. Talanta 99:132–139
Behbahani M, Najafi M, Amini MM, Sadeghi O, Bagheri A, Salarian M (2013a) Dithizone-modified nanoporous fructose as a novel sorbent for solid-phase extraction of ultra-trace levels of heavy metals. Microchim Acta 180:911–920
Behbahani M, Salarian M, Amini MM, Sadeghi O, Bagheri A, Bagheri S (2013b) Application of a new functionalized nanoporous silica for simultaneous trace separation and determination of Cd(II), Cu(II), Ni(II), and Pb(II) in food and agricultural products. Food Anal Methods 6:1320–1329
Behbahani M, Gorji T, Mahyari M, Salarian M, Bagheri A, Shaabani A (2014a) Application of polypropylene amine dendrimers (POPAM)-grafted MWCNTs hybrid materials as a new sorbent for solid-phase extraction and trace determination of gold (III) and palladium (II) in food and environmental samples. Food Anal Method 7:957–966
Behbahani M, Sadeghi Abandansari H, Salarian M, Babapour M, Bagheri A, Nabid MR (2014b) Synthesis and application of a thermosensitive tri-block copolymer as an efficient sample treatment technique for preconcentration and ultra-trace detection of lead ions. Microchim Acta 181:129–137
Brereton RG (1990) Chemometrics. Ellis Horwood, Chichester
Carbonell-Barrachina AA, Garcia E, SanchezSoriano J, Aracil P, Burlo F (2002) Effects of raw materials, ingredients, and production lines on arsenic and copper concentrations in confectionery products. J Agric Food Chem 50:3738–3742
Chen J, Teo KC (2001) Determination of cadmium, copper, lead and zinc in water samples by flame atomic absorption spectrometry after cloud point extraction. Anal Chim Acta 450:215–222
Dong ZP, Dong ZH, Wang P, Tian X, Geng HM, Li R, Ma JT (2010) A fluorescent probe for zinc detection based on organically functionalized SBA-15. Appl Surf Sci 257:802–806
Duran A, Soylak M, Tuncel SA (2008) Poly(vinyl pyridine-poly ethylene glycol methacrylate-ethylene glycol dimethacrylate) beads for heavy metal removal. J Hazard Mater 155(1–2):114–120
Duran A, Tuzen M, Soylak M (2013) Evaluation of metal concentrations in food packaging materials: relation to human health. At Spectrosc 34:99–101
Ebrahimzadeh H, Shekari N, Tavassoli N, Amini MM, Adineh M, Sadeghi O (2010) Extraction of trace amounts of silver on various amino-functionalized nanoporous silicas in real samples. Microchim Acta 170:171–178
Feng XD, Fryxell GE, Wang LQ, Kim AY, Liu J, Kemner KM (1997) Functionalized monolayers on ordered mesoporous supports. Science 276:923–926
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186
Fryxell GE (2006) The synthesis of functional mesoporous materials. Inorg Chem Commun 9:1141–1150
Galan-Cano F, Lucena R, Cardenas S, Valcarcel M (2012) Ionic liquid based in situ solvent formation microextraction coupled to thermal desorption for chlorophenols determination in waters by gas chromatography/mass spectrometry. J Chromatogr A 1229:48–54
Gao ZF, Wang LN, Qi T, Chu JL, Zhang Y (2007) Synthesis, characterization, and cadmium(II) uptake of iminodiacetic acid-modified mesoporous SBA-15. Colloids Surf A Physicochem Eng Asp 304:77–81
Hajiaghababaei L, Badiei A, Ganjali MR, Heydari S, Khaniani Y, Ziarani GM (2011) Highly efficient removal and preconcentration of lead and cadmium cations from water and wastewater samples using ethylenediamine functionalized SBA-15. Desalination 266:182–187
Jeong EY, Ansari MB, Mo YH, Park SE (2011) Removal of Cu(II) from water by tetrakis(4-carboxyphenyl) porphyrin-functionalized mesoporous silica. J Hazard Mater 185:1311–1317
Johnson JS, Stein A (2001) Surface modification of mesoporous, macroporous, and amorphous silica with catalytically active polyoxometalate clusters. Inorg Chem 40:801–808
Kazi TG, Jalbani N, Kazi N, Arain MB, Jamali MK, Afridi HI, Kandehro GA (2008) Evaluation of toxic metals in blood and urine samples of chronic renal failure patients, before and after dialysis. Ren Fail 30:737–745
Liu F, Li KA, Wu YS, Wang X, Tong SY (1995) Study on preconcentration and separation of trace Pd(II) and Pt(IV) with silica gel bonded by aminopropyl-benzoylazo-1-(2 pyridylazo)-2-naphthol. Microchem J 52:274–281
Liu AM, Hidajat K, Kawi S, Zhao DY (2000) A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem Commun 13:1145–1146
Mureseanu M, Reiss A, Stefanescu I, David E, Parvulescu V, Renard G, Hulea V (2008) Modified SBA-15 mesoporous silica for heavy metal ions remediation. Chemosphere 73:1499–1504
Mureseanu M, Reiss A, Cioatera N, Trandafir I, Hulea VJ (2010) Mesoporous silica functionalized with 1-furoyl thiourea urea for Hg(II) adsorption from aqueous media. J Hazard Mater 182:197–203
Nabid MR, Sedghi R, Bagheri A, Behbahani M, Taghizadeh M, Abdi Oskooie H, Heravi MM (2012) Preparation and application of poly(2-amino thiophenol)/MWCNTs nanocomposite for adsorption and separation of cadmium and lead ions via solid phase extraction. J Hazard Mater 203–204:93–100
Parham H, Pourreza N, Rahbar N (2009) Solid phase extraction of lead and cadmium using solid sulfur as a new metal extractor prior to determination by flame atomic absorption spectrometry. J Hazard Mater 163:588–592
Sahmetlioglu E, Yilmaz E, Aktas E, Soylak M (2014) Polypyrrole/multi-walled carbon nanotube composite for the solid phase extraction of lead(II) in water samples. Talanta 119:447–451
Salarian M, Ghanbarpour AR, Behbahani M, Bagheri S, Bagheri A (2014) A metal-organic framework sustained by a nanosized Ag12 cuboctahedral node for solid-phase extraction of ultra traces of lead(II) ions. Microchim Acta 181:999–1007
Soylak M, Tuzen M (2006) Diaion SP-850 resin as a new solid phase extractor for preconcentration-separation of trace metal ions in environmental samples. J Hazard Mater 137(3):1496–1501
Soylak M, Karatepe AU, Elçi L, Doǧan M (2003) Column preconcentration/separation and atomic absorption spectrometric determinations of some heavy metals in table salt samples using Amberlite XAD-1180. Turk J Chem 27(2):235–242
Stalikas C, Fiamegos Y, Sakkas V, Albanis T (2009) Developments on chemometric approaches to optimize and evaluate microextraction. J Chromatogr A 1216:175–189
Teixeira Tarley CR, Silveira G, Lopes dos Santos WN, Matos GD, Paranhos da Silva EG, Bezerra MA, Miro M, Costa Ferreira SL (2009) Chemometric tools in electroanalytical chemistry: methods for optimization based on factorial design and response surface methodology. Microchem J 92:58–67
Tsenga WC, Chenb PS, Huang SD (2014) Optimization of two different dispersive liquid–liquid microextraction methods followed by gas chromatography–mass spectrometry determination for polycyclic aromatic hydrocarbons (PAHs) analysis in water. Talanta 120:425–432
Wang XG, Lin KSK, Chan JCC, Cheng S (2005) Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials. J Phys Chem B 109:1763–1769
Wua X, Yang Y (2011) Heavy metal (Pb, Co, Cd, Cr, Cu, Fe, Mn and Zn) concentrations in harvest-size white shrimp Litopenaeus vannamei tissues from aquaculture and wild source. J Food Compos Anal 24:62–65
Xu J, Luan Z, He H, Zhou W, Kevan L (1998) A reliable synthesis of cubic mesoporous MCM-48 molecular sieve. Chem Mater 10:3690–3698
Zhang J, Lee HK (2010) Headspace ionic liquid-based microdrop liquid-phase microextraction followed by microdrop thermal desorption-gas chromatographic analysis. Talanta 81:537–542
Zhang LX, Yu CC, Zhao WR, Hua ZL, Chen HR, Li L, Shi JL (2007) Preparation of multi-amine-grafted mesoporous silica and their application to heavy metal ions adsorption. J Non-Cryst Solids 353:4055–4061
Zhou HY, Cheung RYH, Chan KM, Wong MH (1998) Metal concentrations in sediments and tilapia collected from inland waters of Hong Kong. Water Res 32:3331–3340
Acknowledgments
We gratefully acknowledge financial support from the Young Researchers and Elite Club, Damghan Branch, Islamic Azad University, Damghan, Iran.
Compliance with Ethics Requirements
ᅟ
Conflict of Interest
Hamid Reza Fouladian declares that he has no conflict of interest. Mohammad Behbahani declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouladian, H.R., Behbahani, M. Solid Phase Extraction of Pb(II) and Cd(II) in Food, Soil, and Water Samples Based on 1-(2-Pyridylazo)-2-Naphthol-Functionalized Organic–Inorganic Mesoporous Material with the aid of Experimental Design Methodology. Food Anal. Methods 8, 982–993 (2015). https://doi.org/10.1007/s12161-014-9981-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-9981-9