Abstract
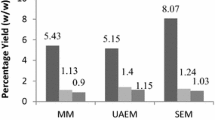
Bauhinia purpurea leaf was extracted by Soxhlet, ultrasonication and maceration extraction methods using ethanol (99.5%, v/v) to obtain Soxhlet (SBE), ultrasonicated (UBE) and macerated (MBE) B. purpurea leaf extract. The effects of different extracting methods on the polyphenolic content and antioxidant activities using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), ferric-reducing antioxidant power (FRAP) and total antioxidant capacity (TAC) were investigated. Disc diffusion and broth dilution methods were also carried out to find the antibacterial activity of these extracts. Findings of this study showed that UBE possessed significant (P < 0.05) polyphenolic constituents followed by MBE and SBE. All the extracts exhibited good DPPH and ABTS radical scavenging as well as potential reducing ability in TAC and FRAP methods. UBE possessed significant (P < 0.05) radical scavenging activity and reducing ability followed by MBE and SBE. Even the results of antibacterial activity were similar to antioxidant activity, with UBE inhibiting most of the bacteria followed by MBE and SBE. All the extracts were subjected to thin layer chromatography (TLC) bioautography followed by high-performance TLC densitometric determination, and the results show that extraction using ultrasonication method yields the highest amount of antioxidant compounds among the three methods mentioned earlier. This study confirms ultrasonic extraction to be an ideal, simple and rapid method to obtain antioxidant- as well as antibacterial-enriched B. purpurea leaf extract. The HPTLC fingerprint profile can be used as a reference data for the standardisation of B. purpurea leaf.



Similar content being viewed by others
References
Asolker LV, Kakkar KK, Chakre OJ (2000) Supplement to glossary of Indian medicinal plants. Part-I (A-K). National Institute of Science Communication, New Delhi, pp 116–117
Bauer AW, Kirby WMM, Sherris JC, Truck M (1966) Antibiotic susceptibility testing by a standardised single disc method. Am J Clin Pathol 45:493–496
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power, the FRAP assay. Anal Biochem 239:70–76
Blois MS (1958) Antioxidant determination by the use of a stable free radical. Nature 181:1199–1200
Boonphong S, Puangsombat P, Baramee A, Mahidol C, Ruchirawat S, Kittakoop P (2007) Bioactive compounds from Bauhinia purpurea possessing antimalarial, antimycobacterial, antifungal, anti-inflammatory and cytotoxic activities. J Nat Prod 70:795–801
Burns RR (1971) Methods for the estimation of tannins in grain, Sorghum. Agron J 63:511–512
Chen SB, Liu HP, Tian RT, Yang DJ, Chen SL, Xu HX, Chan ASC, Xie PS (2006) High-performance thin-layer chromatographic fingerprints of isoflavonoids for distinguishing between Radix puerariae Lobate and Radix puerariae Thomsonii. J Chromatogr A 1121:114–119
Di X, Chan KKC, Leung HW, Huie CW (2003) Fingerprint profiling of acid hydrolyzates of polysachharides extracted from the fruiting bodies and spores of Lingzhi by high-performance thin-layer chromatography. J Chromatogr A 1018:85–95
Elof JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64:711–713
Gu L, Wu T, Wang Z (2009) TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT Food Sci Technol 42:131–136
Hayouni EA, Abedrabba M, Bouix M, Hamdi M (2007) The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem 105:1126–1134
Kirthikar KR, Basu DB (2001) Indian medicinal plants, vol. 4, 2nd edn. Oriental Enterprises, Dehradun, pp 1255–1257
Kuo YH, Yeh MH, Huang SL (1998) A novel 6-butyl-3-hydroxyflavonone from heartwood of Bauhinia purpurea. Phytochemistry 49(8):2529–2530
Muralikrishna KS, Latha KP, Shreedhara CS, Vaidya VP, Krupanidhi AM (2008) Effect of Bauhinia purpurea Linn. on alloxan-induced diabetic rats and isolated frog’s heart. Int J Green Pharm 2(2):83–86
Panda S, Kar A (1999) Withania somnifera and Bauhinia purpurea in the regulation of circulating thyroid hormone concentrations in female mice. J Ethnopharmacol 67:233–239
Penna C, Marino S, Vivot E, Cruanes MC, de D Munoz JD, Cruanes J, Ferraro G, Gutkind G, Martino V (2001) Antimicrobial activity of Argentine plants used in the treatment of infectious diseases: isolation of active compounds from Sebastiania brasiliensis. J Ethnopharmacol 77:37–40
Pettit GR, Numata A, Iwamoto C, Usami Y, Yamada T, Ohishi H, Cragg GM (2006) Antineoplastic agents. 551. isolation and structures of bauhiniastatins 1–4 from Bauhinia purpurea. J Nat Prod 69:323–327
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: specific application to the determination of Vitamin E. Anal Biochem 269:337–341
Proestos C, Komaitis M (2006) Ultrasonically assisted extaction of phenolic compounds from aromatic plants: comparison with conventional extraction technics. J Food Qual 29:567–582
Rajaram N, Janardhanan K (1991) Chemical composition and nutritional potential of tribal pulses Bauhinia purpurea, B. racemosa and B. vahlii. J Agric Food Chem 55:423–431
Ren W, Qiao Z, Wang H, Zhu L, Zhang L (2003) Flavonoids: promising anticancer agents. Med Res Rev 23:519–534
Sakanaka S, Tachibana Y, Okada Y (2005) Preparation and antioxidant of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem 89:569–575
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorizing assay. Free Radic Biol Med 26(9–10):1231–1237
Salatino A, Blatt CTT, Dos Santos DYAC, Vaz AMSF (1999) Foliar flavonoids of nine species of Bauhinia. Rev Bras Bot 22:17–20
Shreedhara CS, Vaidya VP, Vagdevi HM, Latha KP, Muralikrishna KS, Krupanidhi AM (2009) Screening of Bauhinia purpurea Linn. for analgesic and anti-inflammatory activities. Indian J Pharmacol 41(2):75–79
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Soberon JR, Sgariglia MA, Sampietro DA, Quiroga EN, Sierra MG, Vattuone MA (2010) Purification and identification of antibacterial phenolics from Tripodanthus acutifolium leaves. J Appl Microbiol 108:1757–1768
Vijayakumari K, Pugalenthi M, Vadivel V (2007) Effect of soaking and hydrothermal processing methods on the levels of antinutrients and in vitro protein digestibility of Bauhinia purpurea L. seed. Food Chem 103:968–975
Wang L, Weller CL (2006) Recent advances in the extraction of neutraceuticals from plant. Trends Food Sci Technol 17:300–312
Yadava RN, Tripathi P (2000) A novel flavone glycoside from the stem of Bauhinia purpurea. Fitoterapia 71:88–90
Zakaria ZA, Wen LY, Abdul Rahman NI, Abdul Halim AA, Sulaiman MR, Gopalan HK (2007) Antinociceptive, anti-inflammatory and antipyretic properties of the aqueous extract of Bauhinia purpurea leaves in experimental animals. Med Princ Pract 16:443–449
Zakaria ZA (2007) Free radical scavenging activity of some plants available in Malaysia. Iran J Pharmacol Ther 6(1):87–91
Zhou L, Li D, Wang J, Liu Y, Wu J (2007) Antibacterial phenolic compounds from the spines of Gleditsia sinensis Lam. Nat Prod Res 21:283–291
Acknowledgements
This project was funded by USM Research University Grant. The author (Annegowda H.V.) gratefully acknowledges the Institute of Postgraduate Studies of USM, Malaysia, for granting USM Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Annegowda, H.V., Mordi, M.N., Ramanathan, S. et al. Effect of Extraction Techniques on Phenolic Content, Antioxidant and Antimicrobial Activity of Bauhinia purpurea: HPTLC Determination of Antioxidants. Food Anal. Methods 5, 226–233 (2012). https://doi.org/10.1007/s12161-011-9228-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9228-y