Abstract
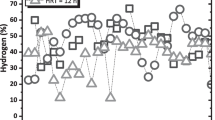
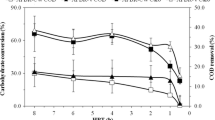
This paper aims to study dark fermentation (DF) in an upflow anaerobic sludge blanket (UASB) reactor during 20 cycles operating at different hydraulic retention times (HRTs) of 3, 9, and 12 h using substrate wastewater from the food industry and granular inoculum sludge from a treatment plant as codigestion to explore the relationship between substrates, metabolites, and microorganisms to increase the biohydrogen (BioH2) yield. Operation conditions were constant pH 5.50 ± 0.50, T = 35 °C and a carbon-to-nitrogen (C/N) ratio of 30. It is noteworthy that DF with HRT = 9 h obtained the highest yields of organic matter degradation, and BioH2 in biogas (~60%) was attributed to the adjustment of pH and the codigestion buffer capacity. During DF with HRT = 9, the reactor was 11 times more productive with regards to HRT = 3 h, resulting in its performance with a better yield mean (6.22 mmol H2 g COD−1) and productivity (0.35 LH2 L−1 d) than other HRTs with lower records of 0.42 mmol H2 g COD−1 and 0.05 LH2 L−1 d, respectively. The high abundance of native populations (Lactobacillus and Lactococcus) and intrinsic inoculum (Citrobacter) led to the highest BioH2 production. Most of the production of acetic acid ~590 mg L−1 and butyric acid ~450 mg L−1 confirmed that BioH2 is mainly produced by acetic and butyric metabolic routes, and a direct relation with the percentage of retention of total suspended solids was also found, supporting the biodegradation capacity of the process.







Similar content being viewed by others
References
Desika P, Varnshini T, Manimudi SSK, Swetha S, Durga MM, Karhik R, Arivalangan P (2018) Advanced biohydrogen production using pretreated industrial waste: outlook and prospects. Renew Sus Energy Rev 96:306–324. https://doi.org/10.1016/j.rser.2018.08.006
Alexandropoulou M, Antonopoulou G, Trably E, Carrere H, Lyberatos G (2018) Continuous biohydrogen production from a food industry waste: influence of operational parameters and microbial community analysis. J Cleaner Prod 174:1054–1063. https://doi.org/10.1016/j.jclepro.2017.11.078
Preisner M, Neverova-Dziopak E, Kowalewski Z (2020) An analytical review of different approaches to wastewater discharge standards with particular emphasis on nutrients. Environ Manag 66:694–708. https://doi.org/10.1007/s00267-020-01344-y
Romão BB, Batista FRX, Ferreira JS, Costa HCB, Resende MM, Cardoso VL (2014) Biohydrogen production through dark fermentation by a microbial consortium using whey permeate as substrate. Appl. Biochem Biotechnol 172:3670–3685. https://doi.org/10.1007/s12010-014-0778-5
Prakash KS, Sonil N (2020) Biohydrogen production through dark fermentation. Chem Eng Technol 43(4):601–612. https://doi.org/10.1002/ceat.201900452
Castelló E, Nunes-Ferraz-Jr AD, Andreani C, Anzola-Rojas MP, Borzacconi L, Buitrón G, Carrillo-Reyes J, Gomes SD, Maintinguer SI, Moreno-Andrade I, Palomo-Briones P, Razo-Flores E, Schiappacasse-Dasati M, Tapia-Venegas E, Valdez-Vázquez I, Vesga-Baron A, Zaiat M, Etchebehere C (2020) Stability problems in the hydrogen production by dark fermentation: possible causes and solutions. Renew Sust Energ Rev 119:109602. https://doi.org/10.1016/j.rser.2019.109602
Villa-Montoya AC, da Silva Mazareli RC, Delforno TP, Centurion VB, de Oliveira VM, Silva EL, Varesche MBA (2020) Optimization of key factors affecting hydrogen production from coffee waste using factorial design and metagenomic analysis of the microbial community. Int J Hydrogen Energy 45(7):4205–4222. https://doi.org/10.1016/j.ijhydene.2019.12.062
Singh V, Yadav S, Sen R, Das D (2020) Concomitant hydrogen and butanol production via co-digestion of organic wastewater and nitrogenous residues. Int J Hydrogen Energy 45:24477–24490. https://doi.org/10.1016/j.ijhydene.2020.06.282
Radjaram B, Saravanane R (2011) Start up study of UASB reactor treating press mud for biohydrogen production. Biomass & Bioenergy 35:2721–2728. https://doi.org/10.1016/j.biombioe.2011.03.016
Mainardis M, Buttazzoni M, Goi D (2022) Up-flow anaerobic sludge blanket (UASB) technology for energy recovery: a review on state-of-the-art and recent technological advances. Bioeng 7:43. https://doi.org/10.3390/bioengineering7020043
Ravichandran P, Balaji K (2020) Effect of HRT on performance of hybrid upflow anaerobic sludge blanket (HUASB) reactor using bio balls in treatment of pulp and paper mill bagasse wash water. Mater Today: Proc 22(2):627–632. https://doi.org/10.1016/j.matpr.2019.09.011
Guozhi D, Xianyang S (2020) The effect of carbon source and COD/N ratio on simultaneous denitrification and methanogenesis in an upflow anaerobic sludge blanket reactor. Renew Energy 157:867–873. https://doi.org/10.1016/j.renene.2020.05.105
Lucena E, De Amorim C, Takano SL, Silva EL (2012) Effect of substrate concentration on dark fermentation hydrogen production using an anaerobic fluidizaed bed reactor. Appl Biochem Biotechnol 166:1248–1263. https://doi.org/10.1007/s12010-011-9511-9
De Gionnanis G, Friargiu M, Massa E, Muntoni A, Polettini A, Pomi R, Spiga D (2014) Biohydrogen production from dark fermentation of cheese whey: influence of pH. Int J Hydrogen Energy 39:20930–20941. https://doi.org/10.1016/j.ijhydene.2014.10.046
Bidattul SZ, Mahmoud D, Noruol SM, Shaliza I (2020) Effect of temperature and dark fermentation effluent on biomethane production in a two-stage up-flow anaerobic sludge fixed-film (UASFF) bioreactor. Fuel 263:116729. https://doi.org/10.1016/j.fuel.2019.116729
Villa Montoya AC, da Silva MRC, Palladino DT, Borin CV, de Oliveira V, Silva EL, Amancio VMB (2022) New insights into controlling hemoacetogenesis in the co-digestion of coffee waste: effect of operational conditions and characterization of microbial communities. Appl Biochem Biotechnol Acepted doi. https://doi.org/10.1007/s120010-021-03725-3
Radjaram B, Saravanane R (2011) Start upstudy of UASB reactor treating press mud biohydrogen production. Biomass Bioenergy 35:2721–2788. https://doi.org/10.1016/j.biombioe.2011.03.016
Buchun S, Jiaming L, Baoming L, Zhangbing Z, Ruixia S, Yuanhui Z, Zhudan L (2015) The role of hydraulic retention time on controlling methanogenesis and homoacetogenesis in biohydrogen production using upflow anaerobic sludge blanket (UASB) reactor and packed bed reactor (PBR). Int J Hydrogen Energy 40:11414–11421. https://doi.org/10.1016/j.ijhydene.2015.04.035
Vassalle L, Díez-Montero R, Trindade A, Machado R, Moreira C, Ferrer I, Mota CR, Passos F (2020) Upflow anaerobic sludge blacket in microalgae-based sewage treatment: co-digestion for improving biogás production. Bioresour Technol 300:122677. https://doi.org/10.1016/j.biortech.2019.122677
Alvarez-Guzmán CL, Cisneros-de la Cueva S, Balderas-Hernández VE, Smoliński A, De León-Rodríguez A (2020) Effect of operating conditions on hydrogen yield and chemometric study of fermentative metabolites. Energy Report 6:1170–1180. https://doi.org/10.1016/j.egyr.2020.04.038
Kim DH, Kim SH, Shin HS (2009) Hydrogen fermentation of food waste without inoculum addition. Enzyme Microb Technol 45:181–187. https://doi.org/10.1016/j.enzmictec.2009.06.013
Anburajan P, Park JH, Sivaguranathan P, Pugazhendhi A, Kumar G, Choi CS, Kim SH (2017) Mixed-culture H2 fermentation performance and the relation between microbial community composition and hydraulic retention times for a fixed bed reactor fed with galactose/glucose mixtures. J Biosci Bioeng 124:339–345. https://doi.org/10.1016/j.jbiosc.2017.04.004
Ahn Y, Lee W, Kang S, Kim SH (2020) Enhancement of sewage sludge digestion by co-digestion with food waste and swine waste. Waste Biomass Valor 11:2421–2430. https://doi.org/10.1007/s12649-018-00558-w
Ramos LR, Silva EL (2018) Continuous hydrogen production from cofermentation of sugarcane vinasse and cheese whey in a thermophilic anaerobic fluidized bed reactor. Int J Hydrogen Energy 43:13081–13089. https://doi.org/10.1016/j.ijhydene.2018.05.070
Ferreira RPR, Santos SC, Silva EL (2014) Different ratios of carbon sources in the fermentation of cheese whey and glucose as substrates for hydrogen and ethanol production in continuous reactors. Int J Hydrogen Energy 39:1288–1296. https://doi.org/10.1016/j.ijhydene.2013.11.011
Szaja A, Montusiewics A (2019) Enhancing the co-digestion efficiency of sewage sludge and cheese whey using brewery spent grain as an additional substrate. Bioresour Technol 291:121863. https://doi.org/10.1016/j.biortech.2019.121863
Reyna-Gómez LM, Molina-Guerrero CE, Alfaro JM, Suárez VSI, Robledo-Olivo A, Cruz-López A (2019) Effect of carbon/nitrogen ratio, temperature, and inoculum source on hydrogen production from dark codigestion of fruit peels and sewage sludge. Sustainability 11:2139. https://doi.org/10.3390/su11072139
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28(3):350–356. https://doi.org/10.1021/ac60111a017
APHA A (2005) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association, and Water Environment Federation.
Reyna-Gómez LM, Cruz-López A, Alfaro JM, Suárez-Vázquez SI (2021) Evaluation of the production of biohydrogen during the co-digestion of organic waste in an upflow hybrid anaerobic reactor. Chemical Eng J 425:129235. https://doi.org/10.1016/j.cej.2021.129235
Náthia-Neves G, Berni M, Dragone G, Mussatto SI, Forster-Carneiro T (2018) Anaerobic digestion process: technological aspect and recent developments. Int J Environ Sci Technol 15:2033–2046. https://doi.org/10.1007/s13762-018-1682-2
Arantes MK, Alves HJ, Sequinel R, da Silva EA (2017) Treatment of brewery wastewater and its use for biological production of methane and hydrogen. Int. J. Hydrogen Energy 42:26243–26256. https://doi.org/10.1016/j.ijhydene.2017.08.206
Sivagurunathan P, Anburajan P, Kumar G, Kim SH (2016) Effect of hydraulic retention time (HRT) on biohydrogen production from galactose in an up-flow anaerobic sludge blanket reactor. Int J Hydrogen Energy 41:21670–21677. https://doi.org/10.1016/j.ijhydene.2016.06.047
Valta K, Damala P, Angeli E, Antonopoulou G, Malamis D, Haralambous KJ (2017) Current treatment technologies of cheese whey and wastewater by Greek cheese manufacturing units and potential valorisation opportunities. Waste Biomass Valorization 8:1649–1663. https://doi.org/10.1007/s12649-017-9862-8
Pérez-Morales J, B-Arroyo C, Morales-Zarate E, Hernándezgarcia H, Méndez-Acosta HO, Hernández-Martínez E (2021) Mathematical modeling of volatile fatty acids production from cheese whey: evaluation of pH and substrate-inoculum ratio effect. Fuel 287:119510. https://doi.org/10.1016/j.fuel.2020.119510
Guanga Y, Jianlong W (2018) Synergistic biohydrogen production from flower waste and sewage sludge, energy fuels. 32:6879-6886. https://doi.org/10.1021/acs.energyfuels.8b01122
Sarkar O, Rova U, Christakopoulos P, Matsakas L (2021) Influence of initial uncontrolled pH on acidogenic fermentation of brewery spent grains to biohydrogen and volatile fatty acids production: optimization and scale-up. Bioresour Technol 319:124233. https://doi.org/10.1016/j.biortech.2020124233
Intanoo P, Rangsanvigit P, Malakul P, Chavadej S (2014) Optimization of separate hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactor (UASB) system under thermophilic operation. Bioresour Technol 173:256–265. https://doi.org/10.1016/j.biortech.2014.09.039
Santos SC, Ferreira Rosa PR, Sakamoto IK, Amâncio Varesche MB, Silva EL (2014) Continuous thermophilic hydrogen production and microbial community analysis from anaerobic digestion of diluted sugar cane stillage. Int. J Hydrogen Energy 39(17):9000–9011. https://doi.org/10.1016/j.ijhydene.2014.03.241
Azbar N, Dokgöz FT, Keskin T, Eltem R, Korkmaz KS, Gezgin Y et al (2009) Comparative evaluation of bio-hydrogen production from cheese whey wastewater under thermophilic and mesophilic anaerobic conditions. Int J Green Energy 6(2):192–200. https://doi.org/10.1080/15435070902785027
Bikram B, Adiba F, Byong-Hun J, Amit G, Pradip KC, Apurba D (2018) Process kinetic studies of biohydrogen production by co-fermentation of fruit-vegetable waste and cottage cheese whey. Energy Sust Develop 47:39–52. https://doi.org/10.1016/j.esd.2018.08.004
Wu H, Dalke R, Mai J, Holtzapple M, Urgun-Demirtas M (2021) Arrested methanogenesis digestion of high-strength cheese whey and Brewery with carboxylic acid production. Bioresour Technol 332:125044. https://doi.org/10.1016/j.biortech.2021.125044
Cabrol L, Marone A, Tapia-Venegas E, Steyer J-P, Ruiz-Filippi G, Trably E (2017) FEMS Microbiol Rev 41:158–181. https://doi.org/10.1093/femsre/fuw043
Sikora A, Blaszczyk M, Jurkowski M, Zielenkiewicz U, (2013) Lactic acid bacteria in hydrogen-producing consortia: on purpose or by coincidence? In: Kongo M (ed.) LAB-R&D for food, Health Livestock Purposes. Rijeka:In TechOpen, 487-514. https://www.intechopen.com/chapters/42319
Castelló E, Nunes-Ferraz-Jr AD, Andreani C, Anzola-Rojas MP, Borzacconi L, Buitrón G, Carrillo-Reyes J, Gomes SD, Maintinguer SI, Moreno-Andrade I, Palomo-Briones P, Razo-Flores E, Schiappacasse-Dasati M, Tapia-Venegas E, Valdez-Vázquez I, Vesga-Baron A, Zaiat M, Etchebehere C (2019) Stability problems in the hydrogen production by dark fermentation: possible causes and solutions. Renew Sust Energy Rev 119:109602. https://doi.org/10.1016/j.rser.2019.109602
Tapia-Venegas E, Cabrol L, Brandhoff B, Hamelin J, Trably E, Steyer JP, Ruiz-Filippi G (2015) Adaptation of acidogenic sludge to increasing glicerol concentrations for biohydrogen production. Appl Microbiol Biotechnol 99:8295–8308. https://doi.org/10.1007/s00253-015-6832-6
Castello E, García C, Santos Iglesias T, Paolino G, Wenzel J, Borzacconi L, Etchebehere C (2009) Feasibility of biohydrogen production from cheese whey using a UASB reactor: links between microbial community and reactor performance. Int J Hydrogen Energy. 34:5674–5682. https://doi.org/10.1016/j.ijhydene.2009.05.060
Acknowledgements
The authors thank the Civil Engineering Faculty, especially the Environmental Engineering Department, for providing its support in the installation of the infrastructure necessary to study bioenergy generation. ACM wishes to thank CONACyT for their support provided through scholarship no. 717875.
Funding
The study has been funded by the CONACyT-SENER-Sustentabilidad Energética provided through Project 249908.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Anaerobic Digestion
Rights and permissions
About this article
Cite this article
Cruz-López, A., Cruz-Méndez, A., Suárez-Vázquez, S.I. et al. Effect of Hydraulic Retention Time on Continuous Biohydrogen Production by the Codigestion of Brewery Wastewater and Cheese Whey. Bioenerg. Res. 17, 1155–1166 (2024). https://doi.org/10.1007/s12155-022-10399-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10399-0