Abstract
The possibility of using waste distillery stillage (first-generation technology) after dilute acid pretreatment, as a medium for the preparation of beet molasses mash, for ethanol production according to the simultaneous saccharification and fermentation (SSF) technology, was assessed. The combination of lignocellulosic hydrolysates made from acid-pretreated stillage with sugar-rich beet molasses is an effective way of utilizing the first-generation ethanol production by-products in the second-generation ethanol production technology. It was demonstrated that the final ethanol concentration could be as high as 90 g/L. The process yield was over 94% of the theoretical yield when the molasses was diluted using acid-pretreated maize distillery stillage. An attempt to increase the pool of fermentable sugars by using cellulases to hydrolyze cellulose failed due to product inhibition in the fermentation medium with a high glucose concentration. A more than threefold increase in the concentration of ethyl acetate (even up to 924.4±11.8 mg/L) was observed in the distillates obtained from the media incubated with cellulases. The use of beet molasses combined with the hydrolysate of pretreated distillery stillage also changed the concentration of other volatile by-products. An increase in the concentration of aldehydes (mainly acetaldehyde to a concentration of above 1500 mg/L), methanol, 1-propanol, and 1-butanol was observed, while the concentration of higher alcohols (isobutanol, 2-methyl-1-butanol, 3-methyl-1-butanol) decreased. Interestingly, the use of cellulases in fermentation media from molasses and stillage hydrolysates resulted in an average fourfold increase in the concentration of this ester to a maximum level of 924.4±11.8 mg/L. Hydrolysates made from acid-pretreated distillery stillage, combined with sugar-rich beet molasses to boost the efficiency of the conversion process, can be successfully used in the production of second-generation fuel ethanol. However, further optimization of the cellulose enzymatic hydrolysis process is required for efficient use of the raw material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Second-generation bioethanol is an alternative to fossil fuels, and its production from lignocellulosic raw materials, which are waste from various branches of the agri-food and wood processing industries, does not pose a risk of rising food prices [1, 2]. The efficient production of cellulosic ethanol by fermentation is highly dependent on the effective pretreatment of lignocellulosic biomass, which reduces the amount of crystalline regions in cellulose and partially degrades hemicelluloses and lignins [3,4,5,6]. These goals can be achieved by developing new or by improving already used methods for the pretreatment of lignocellulose. Numerous methods of biomass pretreatment mainly use dilute inorganic acids (e.g., H2SO4) and elevated pressure on an industrial scale. This method partially hydrolyzes hemicellulose and reduces the crystalline regions in cellulose, making it more susceptible to enzymatic hydrolysis [7]. The high initial sugar concentration needed to obtain an ethanol concentration of at least 40 g/L (the threshold for distillation to be profitable) is most often achieved by thickening the lignocellulosic hydrolysates. However, this has a negative impact on the economics of the process, as significant financial resources are required to remove water from fermentation media [8, 9]. Industrial strains with high fermentation activity used in the production of first-generation ethanol have an increased tolerance to by-products of lignocellulose pretreatment [10, 11]. While each of the abovementioned problems related to the production of second-generation ethanol can be solved individually, only a comprehensive approach to process optimization can lead to technologies that are economically acceptable.
The solution may be to integrate the production of first- and second-generation ethanol into one production line. Previous economic and technological analyses have shown that it is possible to obtain a cost-effective technology for the simultaneous production of fuel ethanol from raw materials containing starch or sucrose and from lignocellulosic materials [12,13,14,15]. The research conducted so far has focused primarily on the development of the integration of first- and second-generation ethanol production technologies from sugar cane or maize, i.e., the two most commonly used raw materials. One of the methods of integration is to run the processes in parallel and then combine the distillation of first- and second-generation ethanol. In this case, the process begins by squeezing the sugar cane juice, then clarifying, and thickening by evaporating the water. The by-product of this stage is sugarcane bagasse, which can be used to produce cellulosic ethanol. The concentrated sugar syrup is then fermented with Saccharomyces cerevisiae yeast. In parallel to this process, the sugarcane bagasse is pretreated; e.g., with the use of dilute acids and increased pressure, cellulose is subjected to enzymatic hydrolysis for about 2–3 days, and the obtained hydrolysate is fermented. Both technological lines meet at the stage of distillation and subsequent dehydration of ethanol [5]. Another way to integrate the production of fuel ethanol from sugar cane is by combining both technologies (first and second generation) at the sugar cane juice thickening stage, i.e., before fermentation. The advantage of this technology is the dilution of the hydrolysate with sugar cane juice after the bagasse pretreatment and the cellulose hydrolysis. Unfortunately, during pretreatment in an acidic environment at elevated pressure and temperature, sugars may dehydrate to fermentation inhibitors such as 5-hydroxymethylfurfural and furfural. The use of sugar cane juice to dilute lignocellulosic hydrolysates with an increased concentration of inhibitors solves the problem of toxic stress during fermentation. An additional production stage that combines both technologies is the use of the waste solid fraction remaining after cellulose hydrolysis for the generation of electricity and steam needed for concentration and distillation operations and the enzymatic hydrolysis process at elevated temperature [16,17,18,19]. A method was also developed to integrate first- and second-generation ethanol production from maize. In this method, maize grain is used to produce ethanol from starch, while the stalks serve as lignocellulosic substrate. Both technological lines also meet at the fermentation stage, as the preparation of raw materials (grinding or pretreatment) and hydrolysis (mashing with the use of amylolytic enzymes and cellulose hydrolysis with cellulases) is carried out separately. The aim of such a technological line is to obtain a fermentation medium with an increased concentration of fermentable sugars (combination of starch and cellulose hydrolysate) and to reduce the concentration of inhibitors generated during the pretreatment of lignocellulose [20, 21].
The main drawback of these solutions is the high energy consumption during the enzymatic hydrolysis of cellulose, which usually lasts for about 48–72 h at ca. 50 °C. The solution to this problem may be the combination of enzymatic hydrolysis and alcoholic fermentation in the SSF (simultaneous saccharification and fermentation) process. The aim of this research was to develop technological assumptions (and to verify them on a laboratory scale) for the production of bioethanol from the by-products of first-generation ethanol production (pretreated distillery stillage) and sucrose from beet molasses. The obtained results allow for a multidimensional assessment of the efficiency of ethanol production from these raw materials using the SSF technology. Due to the specificity of the sugar production process, beet molasses is usually alkaline and contains a large amount of inorganic salts, which makes adjusting the pH of the molasses mash difficult. For this reason, the use of hydrolysate after barothermic treatment of the distillery stillage with the addition of dilute sulfuric acid as a solution for the preparation of molasses mash has been proposed. The proposed concept included the technique for the preparation of such fermentation media and alcoholic fermentation under the conditions of SSF technology with the use of cellulolytic enzymes. The novelty of the proposed solution is the integration of first- and second-generation ethanol production through the use of a hydrolysate from distillery stillage pretreated with dilute acid as a medium for the preparation of beet molasses mash. The combined use of raw materials which are by-products is in line with the current trends in the biotechnological management of postproduction waste. Numerous literature data have shown that the concentration of fermentable sugars in a mash made solely from lignocellulosic biomass is often too low for the process to be profitable. The proposed procedure solves this problem and allows an acceptable level of efficiency of alcoholic fermentation to be obtained.
Materials and Methods
Materials
All reagents (sulfuric acid, sodium hydroxide) used in the study were of analytical purity and were provided by Merck® (Darmstadt, Germany). Chromatographic analyses were performed using high-performance liquid chromatography (HPLC) grade solvents from Merck®. Calibration standards (glucose, fructose, xylose, arabinose, glycerol, ethanol, acetaldehyde, isobutyraldehyde, isovaleraldehyde, furfural, ethyl acetate, methanol, 2-butanol, 1-propanol, isobutanol, 1-butanol, 2-methyl-1-butanol, 3-methyl-1-butanol, 5-hydroxymethylfurfural, 4-hydroxybenzoic acid, vanillin, syringaldehyde, and trans-ferulic acid) for chromatographic analyses were of HPLC grade and were supplied by Sigma-Aldrich® (St. Louis, MO, USA).
Raw Materials
Beet molasses with a sucrose content of 49.70±1.21% and 81.30±0.40° Brix was used in the study. The lignocellulosic raw material used was the distillery stillage (only biomass), obtained by centrifuging rye, wheat, and maize whole stillage. The stillages were obtained from the Radzicz distillery (Poland), which makes use of barothermic technology, where the grain is subjected to high pressure and temperature (6 atm, 160°C) for about 50 min. After enzymatic hydrolysis of starch and fermentation, the mash is distilled to separate the raw spirit. The distillation residue (whole stillage) is centrifuged to obtain a solid fraction which is referred to as a stillage. The distillery stillage biomass was stored at −20°C until the experiments started. The cellulose, hemicellulose, and lignin contents in the distillery stillage samples (expressed on a DW—dry weight basis) were 16.8±0.1%, 29.6±1.5%, and 15.6±0.7% DW in rye stillage; 32.2±1.4%, 20.9±1.2%, and 3.2±1.9% DW in maize stillage; and 18.6±0.3%, 34.1±0.1%, and 9.5±0.2% DW in wheat stillage, respectively [22].
Yeast
Fermentation experiments were carried out using S. cerevisiae yeast strain, namely Ethanol Red (Lesaffre Advanced Fermentations), in the form of an active dried yeast preparation. In the study, the industrial S. cerevisiae strain was used, which is commonly used in the production of first-generation ethanol. The yeast was applied in the form of yeast milk (1.25±0.12 109 CFU—colony-forming unit/mL, viability 94.3±08%) obtained by suspending 5 g of the yeast in 30 mL of sterile 0.9% w/v NaCl followed by rehydration for 30 min (according to the manufacturer’s recommendations). The dose of yeast milk was 3 mL/L of fermentation medium. During each preparation of the starter culture (yeast milk), the viability of the yeast was analyzed and was at a stable level declared by the producer. The viability (%) was estimated after staining with methylene blue and cell counting using Thoma chamber [23].
Cellulolytic Enzymes
Laminex Super 3G (Danisco, DuPont Industrial Biosciences, Archimedesweg 30, Leiden, Netherlands) containing 10–15% complex of beta-glucans hydrolyzing enzymes and non-starch polysaccharides was used for cellulolytic preparation. The enzymatic activity of the preparation at pH = 5 was 35.9 FPU (filter paper units)/mL at 60°C and 18.8 FPU/mL at 35°C, respectively. FPU activity was determined according to the NREL protocol [24].
Preparation of Fermentation Media and Culture Conditions
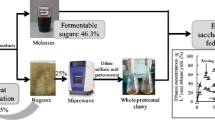
The fermentation media were prepared as shown in Table 1 and Fig. 1. The control contained only beet molasses. The preparation process started by mixing 1000 mL of molasses with 3000 mL of hot tap water (90°C). The resulting solution was kept at reflux for 60 min to pasteurize. After cooling, the pH of the solution was 7.38. The pH of the medium was adjusted to 5 using 25% v/v H2SO4. After cooling to 35°C, the volume was made up to 4000 mL using sterilized tap water. In experimental variants, the media were prepared from molasses with the addition of hydrolysates obtained after pretreatment of rye, maize, and wheat stillage. For the pretreatment, 120-g DW of stillage mixed with 1200 mL of 0.2 M H2SO4 was used. The mixture was then autoclaved (Panasonic MLS 3751L laboratory autoclave) at 131°C for 1 h. Pretreatment parameters were selected as in previous studies [22]. The hydrolysate was made up to 3000 mL with sterile tap water and then mixed with 1000 mL of molasses. The medium prepared in this way was pasteurized at boiling temperature for 60 min. After cooling, the pH of the solution was 4.90 and required only a slight adjustment to 5 with 30% w/v NaOH. The solution was cooled to 35°C, and the volume was adjusted to 4000 mL with sterilized tap water. The fermentation media were poured into an Eppendorf BioFlo 115 bioreactor equipped with a temperature and pH control module, sterile sampling module, and Rushtone stirrer operating at 600 rpm. The yeast inoculum was added at 35°C. In variants where SSF technology was used, cellulase preparation was added at a dose of 5-FPU/g DW. Alcoholic fermentation was carried out for 72 h.
Analytical Methods
Analysis of the Concentration of Lignocellulose Components
Cellulose, hemicellulose, and lignin contents were determined using the FOSS Fibertec® 8000 device. The analysis involved the extraction of NDF (neutral detergent fiber), ADF (acid detergent fiber), and ADL (acid detergent lignin). This was in accordance with the manufacturer’s methodology, ISO 13906:2008 [25] and ISO 16472:2006 [26]. The NDF analysis started with placing 1000±2 mg of Celite 545 (diatomaceous earth) and 500±2 mg of lignocellulosic biomass sample (the measurement was recorded) in sintered ceramic crucibles (P2, 40–100 μm), and 500 ± 100 mg of Na2SO3 was added. Hot extraction was performed using a FOSS Fibertec 8000® instrument with a NDF solution (18.61-g/L C10H14N2Na2O8 × 2H2O; 6.81-g/L Na2B4O7 × 10 H2O; 30-g/L sodium dodecyl sulfate; 10-mL/L C6H14O4; 4.56-g/L Na2HPO4). After extraction, the samples were rinsed with 25 mL of acetone and dried for 2 h at 130°C to constant weight (the measured weight was recorded). The samples were then ashed for 3 h at 525°C (the weight was recorded). Based on the weight difference, the NDF (cellulose, hemicellulose, and lignins) was calculated. The ADF analysis was started by placing 1000±2 mg of Celite 545 and 1000±2 mg of lignocellulosic biomass sample (the weight was recorded) in sintered ceramic crucibles (P2, 40–100 μm). Then, hot extraction was performed using a FOSS Fibertec 8000® instrument with an ADF solution (26.7-mL/L H2SO4 (96%); 20-g/L cetrimonium bromide). After extraction, the samples were rinsed with 25 mL of acetone and dried for 2 h at 130°C to constant weight (the measurement result was recorded). Based on the weight difference, the ADF (cellulose and lignins) was calculated. The residue in the crucibles was used for the determination of ADL and was subjected to acid hydrolysis with 72% v/v H2SO4 for 3 h at room temperature (approx. 22°C). After acid hydrolysis, the sample was rinsed with distilled water until a neutral pH was obtained. The samples were then dried for 2 h at 130°C to constant weight (the result of the measurement was recorded) and ashed for 3 h at 525°C (the measured weight was recorded). Based on the weight difference, the ADF (lignins) was calculated. The cellulose concentration was calculated by subtracting the ADL value from the ADF content. The hemicellulose concentration was calculated by subtracting the ADF value from the NDF content.
Analysis of the Composition of Fermentation Media by HPLC
The composition of fermentation media was tested after inoculation with yeast and after 24, 48, and 72 h of fermentation. Concentrations of glucose, fructose, xylose, arabinose, glycerol, ethanol, acetic acid, and lactic acid were tested by HPLC using the Agilent 1220 device extended with a thermostat module (model 1260) and RID (refractive index detector) (model 1260, Agilent Technologies®, USA) equipped with a Hi-Plex H-Column (7.7 × 300 mm, 8 μm) (Agilent Technologies®, USA). The operating temperature was 60°C for the column and 50°C for the RID. The chromatographic separation was performed with the 5-mM H2SO4 mobile phase at a flow rate of 0.6 mL/min., with an injection of 20 μl (according to the manufacturer’s instructions). Quantitative analyses were performed using the external standards method (ESTD). Before the analysis, the media samples were centrifuged (10 min, 7000 g, 20°C, MPW-260R), and the supernatant was diluted with 5-mM H2SO4 and filtered through a 0.45-μm pore size membrane filter (PES—polyethersulfone). The chromatographic column used did not allow for direct determination of sucrose, the main sugar present in molasses, but did allow the indirect determination of sucrose as the sum of glucose and fructose [27]. Based on the composition of the fermentation medium, the fermentation yield was calculated according to the formula:
where E is the concentration of ethanol (grams per liter), SC is the content of sugars (glucose, fructose, xylose, arabinose in grams per liter), and 0.511 is the value representing the theoretical ethanol yield [28].
Analyzing the Concentration of 5-Hydroxymethylfurfural (5-HMF), Furfural, and Phenol Compounds in Fermentation Media
The concentration of 5-HMF and furfural in the fermentation media was determined using a liquid chromatography system model 1220 by Agilent Technologies®, equipped with a diode detector (HPLC-DAD—diode array detector). The chromatographic separation was performed on a ZORBAX Eclipse Plus C18 column (4.6 × 100 mm, 3.5 μm) (Agilent Technologies®, USA) with a 0.3% acetic acid (70%) and methanol (30%) mobile phase at a flow rate of 0.5 mL/min and temperature of 30°C. Detection of 5-HMF, furfural, 4-hydroxybenzoic acid, and vanillin was performed at 280 nm. Syringaldehyde and trans-ferulic acid were determined at 320 nm [29]. The quantitative analysis was carried out using the external standards method (ESTD).
Analyzing the Composition of Volatile By-products in Raw Spirit by GC (Gas Chromatography) Method
In order to determine the effect of individual process parameters on the metabolic activity of yeast, the concentration of volatile by-products of fermentation in raw spirits after distillation on a glass column equipped with 20 bubble cap trays, under stable distillation conditions, was also analyzed. The ethanol concentration in the distillates was 89.0±0.5% v/v. The raw spirit samples were analyzed by capillary gas chromatography using a model 7890 by Agilent Technologies® equipped with a FID (flame ionization detector) and a CP-Wax 57 CB column (50 m × 0.32 mm, 0.2 μm) (Agilent Technologies®, USA). Detailed conditions of separation are presented in Kłosowski and Mikulski [30].
Statistical Methods
All laboratory analyses were performed in triplicate. Statistical analysis was carried out using the Statistica software ver. 13.3 (analysis of variance, determination of standard deviation, SD). ANOVA test and HSD Tukey’s test (Honest Significant Difference test) were applied at the significance level of α<0.05.
Results and Discussion
Fermentation of Media Composed of Molasses and Stillage Hydrolysates Under the Conditions of the SSF Technology.
The study confirmed the effectiveness of the use of hydrolysates obtained after acidic pretreatment of distillery stillage with 0.2 M H2SO4 at 131°C for the dilution of beet molasses. The use of water for the preparation of a molasses solution requires laborious adjustment of the pH with sulfuric acid to a value of about 5, which is suitable for yeast growth. Replacing the water with the hydrolysate obtained after acid pretreatment with H2SO4 resulted in a pH of about 4.90. The proposed technological solution (fermentation of mixed mash containing pretreated stillage and beet molasses) solves yet another problem in bioethanol production. The use of the acid-pretreated hydrolysate to dilute the alkaline beet molasses eliminates the problematic step of pH adjustment with sulfuric acid to pH 5. The initial total sugar concentration in the medium produced exclusively from beet molasses was 182 g/L and was about 10 g/L statistically significantly lower than in the media with molasses and rye stillage hydrolysates (Fig. 2a). Higher initial sugar concentrations in solutions containing molasses and pretreated stillage hydrolysates were the result of increased concentration of glucose (by ca. 2–5 g/L), xylose (by ca. 1–3 g/L), and arabinose (by ca. 1–2 g/L) (Fig. 2a). The results demonstrated that in each experimental variant, it was possible to carry out an effective alcoholic fermentation in the media containing molasses and distillery stillage hydrolysates. The rate of sugar bioconversion into ethanol varied and depended on the type of stillage (rye, maize, or wheat) and the dose of the cellulolytic enzyme used in SSF technology (Figs. 2 and 3). In the first 48 h of the fermentation process, the rate of glucose and fructose uptake by yeast was similar in the media with only molasses, in the media with molasses and rye stillage hydrolysates, and also in the variant supplemented with cellulases and maize stillage (Fig. 2c). After 72 hours of fermentation, full attenuation (no digestible sugars for yeast) was observed in all experimental variants, except for the combined medium made of molasses and wheat stillage hydrolysate supplemented with cellulolytic enzymes (SSF technology) (Fig. 2d). The final concentration of fermentable sugars in this medium was ca. 10 g/L. The analysis of the absorption rate of fermentable sugars (glucose and fructose) showed that the cellulolytic enzymes contained in Laminex Super 3G had a negative effect on the fermentation activity of yeast. In line with the original research concept, the application of SSF technology and the use of cellulases at the beginning of fermentation were expected to improve the hydrolysis of the cellulosic substrate and increase the concentration of glucose available for fermentation. Surprisingly, a reduction in the rate of sugar assimilation during the first 48 h of the process and incomplete attenuation of media made of molasses and wheat stillage hydrolysates supplemented with cellulases was observed (Fig. 2d). This effect was due to damage to the cell wall caused by the high catalytic activity of cellulases, which, in combination with the osmotic stress, negatively influenced the metabolic activity. The presence of cellulolytic enzymes in the fermentation medium may reduce the viability of yeast (increase in the number of dead cells) and lower the rate of biomass growth [31]. The disturbances observed in the course of fermentation in the media prepared according to the SSF technology were not accidental and were reflected in the concentration of glycerol and ethanol (Fig. 3). Reduced yeast fermentation activity in the media with cellulase resulted in a lower concentration of glycerol (Fig. 3a), and the final concentration of ethanol decreased from 2 to 6 g/L (Fig. 3b) compared to the media containing molasses and stillage hydrolysate in the absence of cellulases. The expected positive effect of addition of cellulase on ethanol concentration was not observed, which could be due to the inhibition of catalytic activity of cellulases by high glucose concentration (product inhibition) [32]. An increased concentration of ethanol in the culture medium can also be the result of a lack of the anticipated effects in the presence of cellulase. The presence of ethanol in concentrations as low as 9% v/v caused a reduction in cellulase activity by up to 30% [33]. It should be noted, however, that higher initial glucose concentrations in media containing molasses and distillery stillage hydrolysates translated into an increase in ethanol concentration by 5.03±0.82 g/L (p<0.001) compared to the medium made of molasses alone (Fig. 3b). The higher concentration of ethanol also resulted in a significantly higher, by ca. 3%, fermentation yield (medium with maize stillage hydrolysate) in relation to the theoretical efficiency calculated for the molasses mash without stillage hydrolysate (Fig. 4). The by-product of the proposed technological solution is a stillage of approximately 5% DW, containing mainly yeast biomass, undecomposed lignocellulose fraction, glycerol, organic acids, and mineral substances, which can be successfully used for biogas production. Alternatively, it may be a component of animal feed mixtures. It can also be composted after mixing with organic materials such as peat, bark, sawdust, or vegetable waste.
The acetic and lactic acid concentrations in the fermentation media were also monitored. After adding stillage hydrolysates to the molasses, there was a slight increase in the acetic acid concentration. A statistically significantly higher initial concentration of acetic acid, by ca. 0.2 g/L (p<0.05), was found in the medium made of molasses and the hydrolysate of maize stillage (Table 2). The acetic acid concentration in all analyzed variants during the entire fermentation process fluctuated around 2 g/L (Table 2). The highest initial concentration of lactic acid, ca. 7.5 g/L, was found in media made of molasses and wheat stillage hydrolysates (Table 2). As a result of yeast metabolic activity, the decrease in the concentration of this acid during fermentation ranged from 0.2 to 1.1 g/L, depending on the experimental variant (Table 2). Furfural and 5-HMF were not found in the fermentation media. These compounds probably evaporated partially during pasteurization for 1 h at a temperature over 95°C. Vanillin, 4-hydroxybenzoic acid, syringaldehyde, and trans-ferulic acid were not found in any of the fermentation samples.
Previous study of authors showed that acid pretreatment of distillery stillage biomass with 0.2 M H2SO4 for 60 min at 131°C was an effective initial step in the production of second-generation ethanol. The yield of ethanol from maize stillage was 60% of the theoretical one [22]. The results of this study confirmed the effectiveness of acid pretreatment in an integrated system for the production of fuel ethanol from distillery stillage hydrolysates enriched with beet molasses. Presumed that the performance of the SSF technology used for the simultaneous fermentation and hydrolysis of the cellulose found in the stillage of the distillery would be sufficient when beginning the research. The aim was to avoid a separate, energy-consuming enzymatic hydrolysis of cellulose lasting up to 72 h at 50°C. Unfortunately, the results of the experiment refuted this assumption. Under the conditions of this study, the SSF technology did not produce the predicted effects due to cellulase activity inhibition (product inhibition). The solution to this problem might be the separation of the cellulose saccharification and fermentation processes proposed by Chen et al. [20] in their work on the use of corn kernels and corn stalks as raw materials for ethanol production in an integrated technology. At the fermentation stage, the obtained starch and cellulose hydrolysates were combined and completely fermented after 72 h. Similar solutions were proposed for sugar cane, but it was also necessary in this case to carry out costly and time-consuming enzymatic hydrolysis of cellulose in addition to a separate fermentation process [18]. Xu et al. [34] also integrated the production of first- and second-generation ethanol by fermenting the mixed mash containing starch and corn stover hydrolysates. Despite the differences in the raw material used and the method of integration, the simultaneous use of starch and cellulose hydrolysate resulted in a high level of glucose conversion into ethanol with 86% fermentation efficiency compared to the theoretical one. In this study, a higher fermentation efficiency (over 94%) was obtained. The possibility of simultaneous fermentation of cellulose hydrolysates in combination with molasses or sugar cane juice, i.e., raw materials used in the production of first-generation ethanol, was also confirmed. The addition of molasses also solves the technological problem associated with the presence of inhibitors in lignocellulosic hydrolysates. In this way, cellulose hydrolysates containing furfural or 5-hydroxymethylfurfural, which can completely inhibit the fermentation process [20], are diluted [18, 19]. The limitations of simultaneous cellulose hydrolysis and fermentation prompt the search for technological solutions aimed at eliminating the inhibition of cellulase products and mitigating the negative effect of cellulolytic enzymes on yeast fermentation activity. One of the possible solutions to be implemented in further stages of the research is fermentation of sugars in the medium before cellulose hydrolysis, which would start only after 48 h of fermentation. Due to the harsh conditions in the fermentation environment, the use of osmoprotectants to support the cellular stress response during the fermentation process is being considered. One of the main benefits of the proposed integrated technology is the use of stillage biomass, a by-product of first-generation ethanol production, to produce second-generation ethanol. The advantages of combining acid-pretreated stillage hydrolysates and sugar-rich beet molasses for the preparation of fermentation media have been presented. One of them is the reduction in the concentration of inhibitors generated at the pretreatment stage. Another advantage is the neutralization of alkaline molasses solutions by acid stillage hydrolysates. The mash pH after combining both ingredients is approximately 5. The addition of stillage hydrolysates increases the concentration of fermentable sugars in the molasses medium by approximately 10 g/L, which results in a statistically higher concentration of ethanol after fermentation compared to the molasses medium without hydrolysates. Other authors [20] proposed the simultaneous use of starch raw materials (corn grains) and lignocellulosic biomass (corn stover) in the production of fuel ethanol. However, due to the high price of the grain, the use of starch raw materials to generate fuel ethanol may not be profitable. This problem can be exacerbated by the low quality of the distillate obtained after combining the starch mash with the cellulose mash. Studies of the authors showed that the distillates from molasses and cellulose hydrolysates are of poorer quality. They contain excessive amounts of volatile fermentation by-products and cannot be for consumption. Therefore, the simultaneous use of molasses and cellulose hydrolysates for the production of fuel ethanol was proposed. Integrating ethanol production through the simultaneous use of beet molasses and acid-pretreated stillage ensured a high fermentation efficiency compared to the theoretical one (up to 94.4%). A comparison of this result with other studies is presented in Table 3. The proposal to produce fuel ethanol from two different raw materials (beet molasses and stillage) is an original contribution to the development of fermentation technology.
Yeast Fermentation Activity and the Composition of Volatile By-products in Samples of Raw Spirits Produced with the Use of SSF Technology
One way to assess the influence of operations and unit processes carried out during ethanol fermentation on the metabolic activity of yeast is to monitor the qualitative and quantitative composition of the volatile by-products generated during the process. Volatile by-products were analyzed in samples of raw spirit obtained by distilling the fermentation media. Although the metabolic activity of yeast can be assessed by the presence of specific groups of volatile by-products of fermentation in the crude spirit, there is no published data showing the composition of volatile by-products in cellulosic distillates. The analysis of the composition of volatile by-products in spirits is important since many countries have standards for the presence of contaminants in fuel ethanol [35, 36]. It should also be noted that the concentration of individual groups of contaminants may vary during the ethanol dehydration process on molecular sieves. Compared to the molasses distillate, the distillates from the molasses/cellulose media had a higher content of aldehydes such as acetaldehyde, isobutyraldehyde, isovaleric aldehyde, and furfural (Fig. 5a). In spirits from distilled fermentation media made of beet molasses and stillage hydrolysates, multiple times higher concentrations of acetaldehyde were identified. The increase was threefold for the rye stillage and more than fivefold for the media made from molasses and maize or wheat stillage hydrolysates (Fig. 5a). This clearly indicates that the activity of alcohol dehydrogenase, which is a key enzyme involved in the reduction of acetaldehyde to ethanol, is expected to decrease when inhibitors such as furan aldehydes are present in hydrolysates after acid pretreatment of lignocellulose [37]. The analysis of changes in the concentration of ethyl acetate in distillates in experimental variants showed that the concentration of this compound in the distillates obtained from molasses-cellulose media without cellulases was lower by more than 120 mg/L, compared to pure molasses distillate. Interestingly, the use of cellulases in fermentation media from molasses and stillage hydrolysates resulted in an average fourfold increase in the concentration of this ester to the maximum level of 924.4±11.8 mg/L (p<0.001) (Fig. 5b). The use of highly active cellulases may destabilize the yeast cell wall and, as a result, affect cell homeostasis. This in turn disrupts the cellular metabolism of yeast and may increase the activity of acetyltransferase, the enzyme involved in the formation of ethyl acetate, during alcohol fermentation [38]. The concentrations of methanol, 1-butanol, and 1-propanol in the distillates from molasses and stillage hydrolysates were also higher than in the molasses distillates (Fig. 5b, 5c). The increased amount of methanol is not surprising as this compound comes from the breakdown of pectins that are not present in the molasses. The higher concentration of 1-propanol in the molasses-cellulose distillates is likely due to the higher abundance of fermentable sugars that are involved in the biosynthesis of this higher alcohol, which has no amino acid precursor [39]. In contrast to the abovementioned alcohols, the molasses-cellulose distillates contained lower concentrations of isobutanol, 2-methyl-1-butanol, and 3-methyl-1-butanol compared to molasses distillates. The concentrations of isobutanol, 2-methyl-1-butanol, and 3-methyl-1-butanol were lower by ca. 180 mg/L (p<0.001), 230 mg/L (p<0.001), and 190 mg/L (p<0.001), respectively (Fig. 5c). This shows that the by-products of acid lignocellulose pretreatment are toxic and they slow down the metabolism of valine, isoleucine, and leucine, which are the precursors to these higher alcohols [40]. The reduced concentration of higher alcohols in the distillates may be caused not only by the limited amount of the corresponding amino acids but also by the presence of inhibitors or by a damage to branched-chain amino acid transaminase, a key enzyme involved in the biosynthesis of isobutanol, 2-methyl-1-butanol, and 3-methyl-1-butanol [41]. The lowest concentration of the abovementioned higher alcohols was found in the distillates obtained from the media with molasses and wheat stillage hydrolysate. In these media, the highest concentration of lactic acid (ca. 7.5 g/L) was found; therefore, this compound was probably responsible for the reduced activity of the enzyme involved in the biosynthesis of isobutanol, 2-methyl-1-butanol, and 3-methyl-1-butanol.
Conclusions
The use of beet molasses and waste distillery stillage after dilute acid pretreatment at 131°C in one technological line is an alternative way of integrating the production of first- and second-generation fuel ethanol. The hydrolysates obtained from the acidic pretreatment of distillery stillage can be combined with alkaline molasses to provide a complete fermentation medium. However, the proposed method of integrating the first- and second-generation ethanol production using SSF technology requires further optimization in order to maintain high catalytic activity of cellulases for a more efficient use of the raw material. The results obtained indicate the further course of research aimed at developing an efficient technology for the production of cellulosic ethanol from agri-food industry waste materials.
References
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38:449–467. https://doi.org/10.1016/j.pecs.2012.03.002
Zabed H, Sahu JN, Suely A, Boyce AN, Faruq G (2017) Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sustain Energy Rev 71:475–501. https://doi.org/10.1016/j.rser.2016.12.076
Galbe M, Zacchi G (2012) Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass Bioenerg 46:70–78. https://doi.org/10.1016/j.biombioe.2012.03.026
Geddes CC, Peterson JJ, Roslander C, Zacchi G, Mullinnix MT, Shanmugam KT, Ingram LO (2010) Optimizing the saccharification of sugar cane bagasse using dilute phosphoric acid followed by fungal cellulases. Bioresource Technol 101:1851–1857. https://doi.org/10.1016/j.biortech.2009.09.070
Macrelli S, Galbe M, Wallberg O (2014) Effects of production and market factors on ethanol profitability for an integrated first and second generation ethanol plant using the whole sugarcane as feedstock. Biotechnol Biofuels 7:26. https://doi.org/10.1186/1754-6834-7-26
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37:52–68. https://doi.org/10.1016/j.pecs.2010.01.003
Camasasca L, Ramirez MB, Guigou M, Ferrari MD, Lareo C (2015) Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of napiergrass for fuel ethanol production. Biomass Bioenerg 74:193–201. https://doi.org/10.1016/j.biombioe.2015.01.017
Xu F, Sun J, Konda NM, Shi J, Dutta T, Scown CD, Simmons BA, Singh S (2016) Transforming biomass conversion with ionic liquids: process intensification and the development of a high-gravity, one-pot process for the production of cellulosic ethanol. Energy Environ Sci 9:1042–1049. https://doi.org/10.1039/C5EE02940F
Xu Y, Wang D (2017) Integrating starchy substrate into cellulosic ethanol production to boost ethanol titers and yields. Appl Energ 195:196–203. https://doi.org/10.1016/j.apenergy.2017.03.035
Kumari R, Pramanik K (2012) Improvement of multiple stress tolerance in yeast strain by sequential mutagenesis for enhanced bioethanol production. J Biosci Bioeng 114(6):622–629. https://doi.org/10.1016/j.jbiosc.2012.07.007
Moon J, Liu ZL (2012) Engineered NADH-dependent GRE2 from Saccharomyces cerevisiae by directed enzyme evolution enhances HMF reduction using additional cofactor NADPH. Enzyme Microb Tech 50:115–120. https://doi.org/10.1016/j.enzmictec.2011.10.007
Akgul O, Shah N, Papageorgiou LG (2012) An optimisation framework for a hybrid first/second generation bioethanol supply chain. Comput Chem Eng 42:101–114. https://doi.org/10.1016/j.compchemeng.2012.01.012
Dias MOS, Junqueira TL, Cavalett O, Pavanello LG, da Cunha MP, Jesus CDF, Filho RM, Bonomi A (2013) Biorefineries for the production of first and second generation ethanol and electricity from sugarcane. Appl Energ 109:72–78. https://doi.org/10.1016/j.apenergy.2013.03.081
Furlan FF, Costa CBB, Fonseca GC, Soares RP, Secchi AR, Cruz AJG, Giordano RC (2012) Assessing the production of first and second generation bioethanol from sugarcane through the integration of global optimization and process detailed modeling. Comput Chem Eng 43:1–9. https://doi.org/10.1016/j.compchemeng.2012.04.002
Miret C, Chazara P, Montastru L, Negny S, Domenech S (2016) Design of bioethanol green supply chain: comparison between first and second generation biomass concerning economic, environmental and social criteria. Comput Chem Eng 85:16–35. https://doi.org/10.1016/j.compchemeng.2015.10.008
Dias MOS, Junqueira TL, Rossell CEV, Filho RM, Bonomi A (2013) Evaluation of process configurations for second generation integrated with first generation bioethanol production from sugarcane. Fuel Process Technol 109:84–89. https://doi.org/10.1016/j.fuproc.2012.09.041
Dias MOS, Junqueira TL, Cavalett O, Cunha MP, Jesus CDF, Rossell CEV, Filho RM, Bonomi A (2012) Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresource Technol 103:152–161. https://doi.org/10.1016/j.biortech.2011.09.120
Dias MOS, da Cunha MP, Filho RM, Bonomi A, Jesus CDF, Rossell CEV (2011) Simulation of integrated first and second generation bioethanol production from sugarcane: comparison between different biomass pretreatment methods. J Ind Microbiol Biotechnol 38:955–966. https://doi.org/10.1007/s10295-010-0867-6
Oliveira CM, Cruz AJG, Costa CBB (2016) Improving second generation bioethanol production in sugarcane biorefineries through energy integration. Appl Therm Eng 109:819–827. https://doi.org/10.1016/j.applthermaleng.2014.11.016
Chen S, Xu Z, Li X, Yu J, Cai M, Jin M (2018) Integrated bioethanol production from mixtures of corn and corn stover. Bioresource Technol 258:18–25. https://doi.org/10.1016/j.biortech.2018.02.125
Lennartsson PR, Erlandsson P, Taherzadeh MJ (2014) Integration of the first and second generation bioethanol processes and the importance of by-products. Bioresource Technol 165:3–8. https://doi.org/10.1016/j.biortech.2014.01.127
Mikulski D, Kłosowski G (2018) Efficiency of dilute sulfuric acid pretreatment of distillery stillage in the production of cellulosic ethanol. Bioresource Technol 268:424–433. https://doi.org/10.1016/j.biortech.2018.08.005
Alfenore S, Molina-Jouve C, Guillouet SE, Uribelarrea J-L, Goma G, Benbadis L (2002) Improving ethanol production and viability of Saccharomyces cerevisiae by a vitamin feeding strategy during fed-batch process. Appl Microbiol Biotechnol 60:67–72
Adney B, Baker J (1996) NREL/TP-510-42628 Technical Report. Measurement of cellulase activities, Laboratory Analytical Procedure
ISO 13906:2008 Animal feeding stuffs – determination of acid detergent fibre (ADF) and acid detergent lignin (ADL) contents.
ISO 16472:2006 Animal feeding stuffs – determination of amylase-treated neutral detergent fibre content (aNDF).
Kłosowski G, Mikulski D (2018) Complementarity of the raw material composition of very high gravity (VHG) mashes as a method to improve efficiency of the alcoholic fermentation process. Process Biochem 74:1–9. https://doi.org/10.1016/j.procbio.2018.08.028
Berłowska J, Pielech-Przybylska K, Balcerek M, Dziekońska-Kubczak U, Patelski P, Dziugan P, Kręgiel D (2016) Simultaneous Saccharification and Fermentation of Sugar Beet Pulp for Efficient Bioethanol Production. In: Simultaneous saccharification and fermentation of sugar beet pulp for efficient bioethanol production. BioMed Research International https://doi.org/10.1155/2016/3154929
Cho DH, Lee YJ, Um Y, Sang B-I, Kim YH (2009) Detoxification of model phenolic compounds in lignocellulosic hydrolysates with peroxidase for butanol production from Clostridium beijerinckii. Appl Microbiol Biotechnol 83:1035–1043. https://doi.org/10.1007/s00253-009-1925-8
Kłosowski G, Mikulski D (2010) The effect of raw material contamination with mycotoxins on the composition of alcoholic fermentation volatile by-products in raw spirits. Bioresource Technol 101:9723–9727. https://doi.org/10.1016/j.biortech.2010.07.085
Tomás-Pejó E, García-Aparicio M, Negro MJ, Oliva JM, Ballesteros M (2009) Effect of different cellulase dosages on cell viability and ethanol production by Kluyveromyces marxianus in SSF processes. Bioresource Technol 100(2):890–895. https://doi.org/10.1016/j.biortech.2008.07.012
Teugjas H, Väljamäe P (2013) Product inhibition of cellulases studied with 14C-labeled cellulose substrates. Biotechnol Biofuels 6:104. https://doi.org/10.1186/1754-6834-6-104
Chen H, Jin S (2006) Effect of ethanol and yeast on cellulase activity and hydrolysis of crystalline cellulose. Enzyme Microb Tech 39(7):1430–1432. https://doi.org/10.1016/j.enzmictec.2006.03.027
Xu Y, Zhang M, Roozeboom K, Wang D (2018) Integrated bioethanol production to boost low-concentrated cellulosic ethanol without sacrificing ethanol yield. Bioresource Technol. 250:299–305. https://doi.org/10.1016/j.biortech.2017.11.056
EN 15376:2014, Automotive fuels. Ethanol as a blending component for petrol. Requirements and test methods.
ASTM (2020) D5798-20, Standard specification for ethanol fuel blends for flexible-fuel automotive spark-ignition engines. ASTM International, West Conshohocken, PA https://doi.org/10.1520/D5798-20
Modig T, Liden G, Taherzadeh MJ (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776. https://doi.org/10.1042/0264-6021:3630769
Malcorps P, Cheval JM, Jamil S, Dufour JP (1991) A new model for the regulation of ester synthesis by alcohol acetyltransferase in Saccharomyces cerevisiae during fermentation. J Am Sot Brew Chem 49:47–53. https://doi.org/10.1094/ASBCJ-49-0047
Cachot T, Müller M, Pons M-N (1991) Kinetics of volatile metabolites during alcoholic fermentation of cane molasses by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 35:450–454. https://doi.org/10.1007/BF00169748
Hazelwood LA, Daran J-M, van Maris AJA, Pronk JT, Dickinson R (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microb 74(8):2259–2266. https://doi.org/10.1128/AEM.02625-07
Lilly M, Bauer FF, Styger G, Lambrechts MG, Pretorius IS (2006) The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res 6(5):726–743. https://doi.org/10.1111/j.1567-1364.2006.00057.x
Funding
This study was supported by the Polish Minister of Science and Higher Education, under the program “Regional Initiative of Excellence” in 2019–2022 (Grant No. 008/RID/2018/19).
Author information
Authors and Affiliations
Contributions
DM conceptualization, methodology, validation, formal analysis, investigation, resources, writing—original draft, and funding acquisition. GK conceptualization, formal analysis, writing—review and editing, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mikulski, D., Kłosowski, G. Integration of First- and Second-generation Bioethanol Production from Beet molasses and Distillery Stillage After Dilute Sulfuric Acid Pretreatment. Bioenerg. Res. 15, 454–465 (2022). https://doi.org/10.1007/s12155-021-10260-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10260-w